-- While energy drinks are promoted as products that increase energy and enhance performance, they also can put users at risk.
From 2007 to 2011, energy-drink related emergency room visits doubled. Overconsumption of energy drinks can cause a host of i

FDA Approves Spravato (esketamine) Nasal Spray for Treatment-Resistant Depression
March 5, 2019 -- The U.S. Food and Drug Administration today approved Spravato (esketamine) nasal spray, in conjunction with an oral antidepressant, for the treatment of de

AHA News: Is Long-Distance Running Good for the Heart?
FRIDAY, March 1, 2019 (American Heart Association News) -- As you can tell by all those 26.2-mile bumper stickers popping up around the country, the popularity of marathons and long-distance running

FDA Approves Herceptin Hylecta
FDA Approves Herceptin Hylecta for Subcutaneous Injection in Certain HER2-Positive Breast Cancers
South San Francisco, CA -- February 28, 2019 -- Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), today

FDA Approves Adhansia XR
FDA Approves Adhansia XR (methylphenidate hydrochloride) Extended-Release Capsules for the Treatment of ADHD
STAMFORD, Conn.--(BUSINESS WIRE) March 01, 2019 --Adlon Therapeutics L.P., a subsidiary of Purdue Pharma L.P., announ


 Deutsche
Deutsche Español
Español français
français italiano
italiano português
português 日本語
日本語 한국어
한국어 العربية
العربية русский
русский bahasa Indonesia
bahasa Indonesia Tiếng Việt
Tiếng Việt
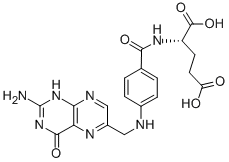 Folic acid,59-30-3
Folic acid,59-30-3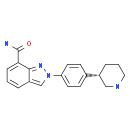 MK-4827 (HCl)
MK-4827 (HCl)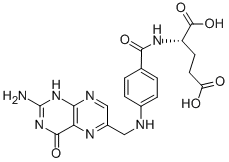 Folic acid
Folic acid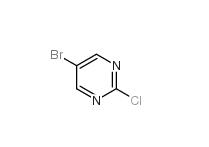 5-Bromo-2-chloropyrimidine 32779-36-5
5-Bromo-2-chloropyrimidine 32779-36-5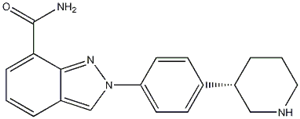 Niraparib
Niraparib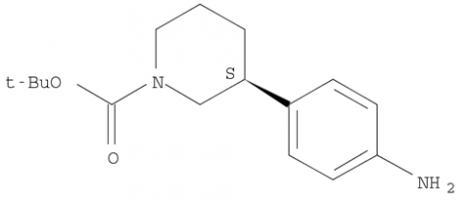 (R)-tert-butyl 3-(4-aminophenyl)piperidine-1-carboxylate
(R)-tert-butyl 3-(4-aminophenyl)piperidine-1-carboxylate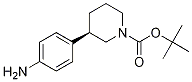 (R)-tert-butyl 3-(4-aMinophenyl)piperidine-1-carboxylate
(R)-tert-butyl 3-(4-aMinophenyl)piperidine-1-carboxylate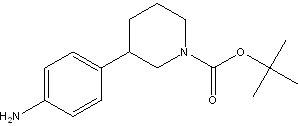 tert-butyl 3-(4-aminophenyl)piperidine-1-carboxylate
tert-butyl 3-(4-aminophenyl)piperidine-1-carboxylate![(3S)-3-[4-[7-[[(1,1-Dimethylethyl)amino]carbonyl]-2H-indazol-2-yl]phenyl]-1-piperidinecarboxylic acid 1,1-dimethylethyl ester (3S)-3-[4-[7-[[(1,1-Dimethylethyl)amino]carbonyl]-2H-indazol-2-yl]phenyl]-1-piperidinecarboxylic acid 1,1-dimethylethyl ester](/data/attachment/201705/26/3446bd2b841689a5afc36447418dc476.png) (3S)-3-[4-[7-[[(1,1-Dimethylethyl)amino]carbonyl]-2H-indazol-2-yl]phenyl]-1-piperidinecarboxylic acid 1,1-dimethylethyl ester
(3S)-3-[4-[7-[[(1,1-Dimethylethyl)amino]carbonyl]-2H-indazol-2-yl]phenyl]-1-piperidinecarboxylic acid 1,1-dimethylethyl ester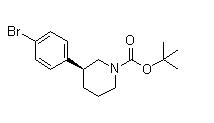 (3S)-3-(4-Bromophenyl)-1-piperidinecarboxylic acid 1,1-dimethylethyl ester
(3S)-3-(4-Bromophenyl)-1-piperidinecarboxylic acid 1,1-dimethylethyl ester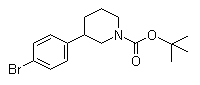 3-(4-Bromophenyl)piperidine-1-carboxylic acid tert-butyl ester
3-(4-Bromophenyl)piperidine-1-carboxylic acid tert-butyl ester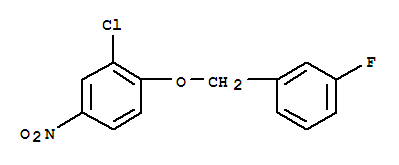 3-Chloro-4-(3-fluorobenzyloxy)nitrobenzene
3-Chloro-4-(3-fluorobenzyloxy)nitrobenzene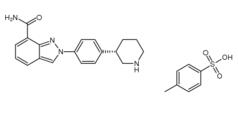 Niraparib p-toluenesulfonate
Niraparib p-toluenesulfonate![N-(1,1-Dimethylethyl)-2-[4-(3S)-3-piperidinylphenyl]-2H-indazole-7-carboxamide N-(1,1-Dimethylethyl)-2-[4-(3S)-3-piperidinylphenyl]-2H-indazole-7-carboxamide](/data/attachment/201705/26/da41ae70b523a458db70333bd1059362.png) N-(1,1-Dimethylethyl)-2-[4-(3S)-3-piperidinylphenyl]-2H-indazole-7-carboxamide
N-(1,1-Dimethylethyl)-2-[4-(3S)-3-piperidinylphenyl]-2H-indazole-7-carboxamide![2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide](/data/attachment/201705/26/d3114dd994f3dda3142cba7d326bcede.jpg) 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide
2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide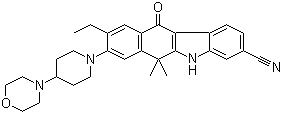 Alectinib
Alectinib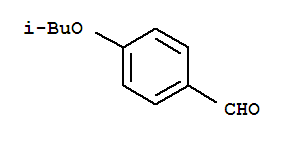 Benzaldehyde,4-(2-methylpropoxy)
Benzaldehyde,4-(2-methylpropoxy)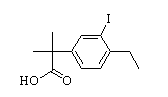 2-(4-ethyl-3-iodophenyl)-2-Methylpropanoic acid
2-(4-ethyl-3-iodophenyl)-2-Methylpropanoic acid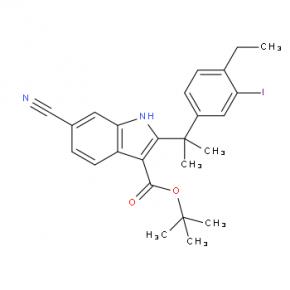 tert-butyl 6-cyano-2-(2-(4-ethyl-3-iodophenyl)propan-2-yl)-1H-indole-3-carboxylate
tert-butyl 6-cyano-2-(2-(4-ethyl-3-iodophenyl)propan-2-yl)-1H-indole-3-carboxylate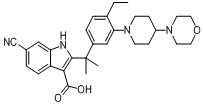 6-cyano-2-(2-(4-ethyl-3-(4-morpholinopiperidin-1-yl)phenyl)propan-2-yl)-1H-indole-3-carboxylic acid
6-cyano-2-(2-(4-ethyl-3-(4-morpholinopiperidin-1-yl)phenyl)propan-2-yl)-1H-indole-3-carboxylic acid![9-ethyl-8-iodo-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile 9-ethyl-8-iodo-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile](/data/attachment/201705/28/e48e5d316800efe6192ebfdeec6cf28c.gif) 9-ethyl-8-iodo-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile
9-ethyl-8-iodo-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile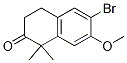 6-broMo-7-Methoxy-1,1-diMethyl-3,4-dihydronaphthalen-2(1H)-one
6-broMo-7-Methoxy-1,1-diMethyl-3,4-dihydronaphthalen-2(1H)-one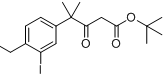 tert-butyl 4-(4-ethyl-3-iodophenyl)-4-methyl-3-oxopentanoate
tert-butyl 4-(4-ethyl-3-iodophenyl)-4-methyl-3-oxopentanoate![9-broMo-8-hydroxy-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile 9-broMo-8-hydroxy-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile](/data/attachment/201705/28/fe98529212eb834b17a38f13138a35bf.png) 9-broMo-8-hydroxy-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile
9-broMo-8-hydroxy-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile![9-ethyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11a-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride 9-ethyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11a-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride](/data/attachment/201705/28/36e5363f0c9f92378b75195743e2abb2.jpg) 9-ethyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11a-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride
9-ethyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11a-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride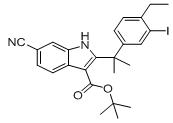 tert-butyl 6-cyano-2-(2-(4-ethyl-3-iodophenyl)propan-2-yl)-1H-indole-3-carboxylate
tert-butyl 6-cyano-2-(2-(4-ethyl-3-iodophenyl)propan-2-yl)-1H-indole-3-carboxylate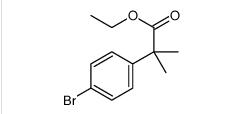 ethyl 2-(4-broMophenyl)-2-Methylpropanoate
ethyl 2-(4-broMophenyl)-2-Methylpropanoate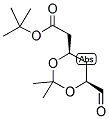 ert-Butyl (4R-cis)-6-formaldehydel-2,2-dimethyl-1,3-dioxane-4-acetate
ert-Butyl (4R-cis)-6-formaldehydel-2,2-dimethyl-1,3-dioxane-4-acetate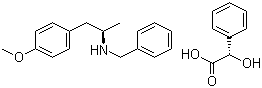 (2S)-Hydroxy(phenyl)acetic acid (2R)-N-benzyl-1-(4-methoxyphenyl)propan-2-amine
(2S)-Hydroxy(phenyl)acetic acid (2R)-N-benzyl-1-(4-methoxyphenyl)propan-2-amine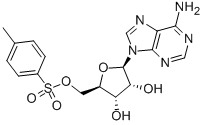 5-Tosyladenosine
5-Tosyladenosine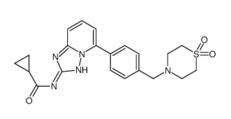 Filgotinib
Filgotinib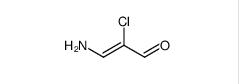 3-amino-2-chloroacrolein
3-amino-2-chloroacrolein![2-Methylpyrazolo[1,5-a]pyrimidine-6-carboxamide 2-Methylpyrazolo[1,5-a]pyrimidine-6-carboxamide](/data/attachment/201706/03/2e19d959128718d26901f9909d7b9342.jpg) 2-Methylpyrazolo[1,5-a]pyrimidine-6-carboxamide
2-Methylpyrazolo[1,5-a]pyrimidine-6-carboxamide![11-(1-Methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[d]iMidazo[1,2-a]azepine 11-(1-Methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[d]iMidazo[1,2-a]azepine](/data/attachment/201706/03/1549d9affee63ead337049001f25d9fa.jpg) 11-(1-Methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[d]iMidazo[1,2-a]azepine
11-(1-Methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[d]iMidazo[1,2-a]azepine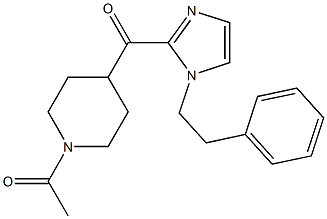 1-(4-(1-PHENETHYL-1H-IMIDAZOLE-2-CARBONYL)PIPERIDIN-1-YL)ETHANONE
1-(4-(1-PHENETHYL-1H-IMIDAZOLE-2-CARBONYL)PIPERIDIN-1-YL)ETHANONE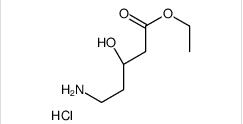 ethyl (3R)-5-amino-3-hydroxypentanoate,hydrochloride
ethyl (3R)-5-amino-3-hydroxypentanoate,hydrochloride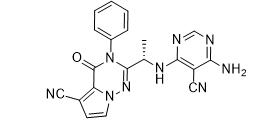 LAS191954 free base
LAS191954 free base![tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate](/data/attachment/201706/03/8a3c0fcdeb9ed744fc854cf248d4d53e.jpg) tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate
tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate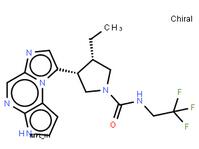 ABT-494 Intermeidate N-2
ABT-494 Intermeidate N-2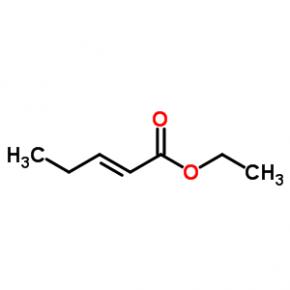 ethyl (2E)-pent-2-enoate
ethyl (2E)-pent-2-enoate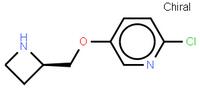 abt594 Intermediate
abt594 Intermediate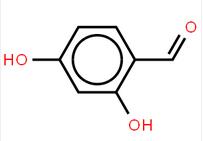 LOXO101 Intermediate 2
LOXO101 Intermediate 2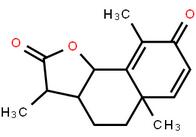 LOXO101 Intermediate 1
LOXO101 Intermediate 1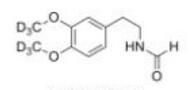 Deutetrabenazine intermediate N-2
Deutetrabenazine intermediate N-2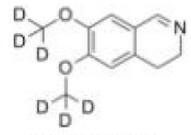 Deutetrabenazine intermediate N-1
Deutetrabenazine intermediate N-1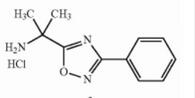 Naldemedine tosylate intermediate
Naldemedine tosylate intermediate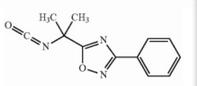 Naldemedine tosylate intermediate N-2
Naldemedine tosylate intermediate N-2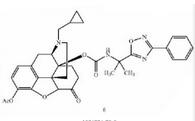 Naldemedine
Naldemedine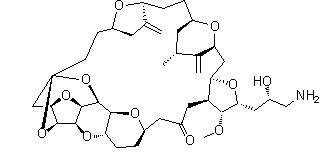 Eribulin
Eribulin![2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]tetrahydro-4-methylene-, 2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]tetrahydro-4-methylene-,](/data/attachment/201706/03/3575f40dcc389832ca73cc99972a645b.gif.thumb.jpg) 2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]tetrahydro-4-methylene-,
2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]tetrahydro-4-methylene-,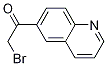 2-BroMo-1-quinolin-6-yl-ethanone
2-BroMo-1-quinolin-6-yl-ethanone![6-[(6-Bromo-1H-[1,2,3]triazolo[4,5-b]pyrazin-1-yl)methyl]-7-fluoroquinoline 6-[(6-Bromo-1H-[1,2,3]triazolo[4,5-b]pyrazin-1-yl)methyl]-7-fluoroquinoline](/data/attachment/201706/07/27ae4307b53f4294590fb8f914894490.jpg) 6-[(6-Bromo-1H-[1,2,3]triazolo[4,5-b]pyrazin-1-yl)methyl]-7-fluoroquinoline
6-[(6-Bromo-1H-[1,2,3]triazolo[4,5-b]pyrazin-1-yl)methyl]-7-fluoroquinoline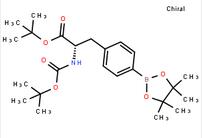 tert-butyl (S)-2-((tert-butoxycarbonyl)amino)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)propanoate
tert-butyl (S)-2-((tert-butoxycarbonyl)amino)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)propanoate![7-Trifluoromethyl-imidazo[1,2-a]pyridine 7-Trifluoromethyl-imidazo[1,2-a]pyridine](/data/attachment/201706/07/24ba6100528abe0753ad9e82ef8dc810.gif) 7-Trifluoromethyl-imidazo[1,2-a]pyridine
7-Trifluoromethyl-imidazo[1,2-a]pyridine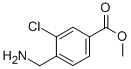 methyl 4-(aminomethyl)-3-chlorobenzoate
methyl 4-(aminomethyl)-3-chlorobenzoate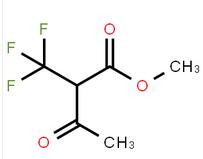 methyl 3-oxo-2-(trifluoromethyl)butanoate
methyl 3-oxo-2-(trifluoromethyl)butanoate![2-AMino-4,5,6,7-tetrahydro-benzo[b]thiophene-3-carboxylic acid tert-butyl ester 2-AMino-4,5,6,7-tetrahydro-benzo[b]thiophene-3-carboxylic acid tert-butyl ester](/data/attachment/201706/07/22aadd4c55094254a681014935f56827.jpg) 2-AMino-4,5,6,7-tetrahydro-benzo[b]thiophene-3-carboxylic acid tert-butyl ester
2-AMino-4,5,6,7-tetrahydro-benzo[b]thiophene-3-carboxylic acid tert-butyl ester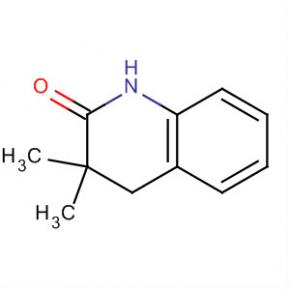 2(1H)-Quinolinone, 3,4-dihydro-3,3-dimethyl
2(1H)-Quinolinone, 3,4-dihydro-3,3-dimethyl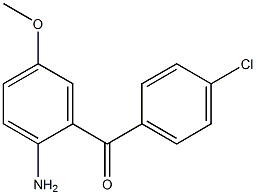 Methanone, (2-aMino-5-Methoxyphenyl)(4-chlorophenyl)
Methanone, (2-aMino-5-Methoxyphenyl)(4-chlorophenyl)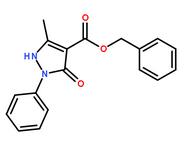 benzyl 5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxylate
benzyl 5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxylate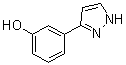 3-(1H-pyrazol-5-yl)phenol
3-(1H-pyrazol-5-yl)phenol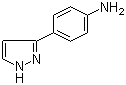 4-(1H-Pyrazol-3-yl)aniline
4-(1H-Pyrazol-3-yl)aniline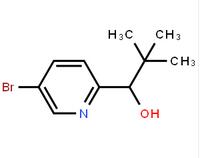 1-(5-bromo-pyridin-2-yl)-2,2-dimethyl-propan-1-ol
1-(5-bromo-pyridin-2-yl)-2,2-dimethyl-propan-1-ol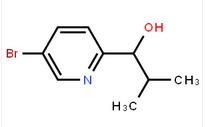 1-(5-bromo-pyridin-2-yl)-2-methyl-propan-1-ol
1-(5-bromo-pyridin-2-yl)-2-methyl-propan-1-ol![Benzenemethanol, a-[(1S)-1-aminoethyl]-4-hydroxy-,(aR) Benzenemethanol, a-[(1S)-1-aminoethyl]-4-hydroxy-,(aR)](/data/attachment/201706/07/c4adcbada0ef372ae46cbaed643dd18e.jpg) Benzenemethanol, a-[(1S)-1-aminoethyl]-4-hydroxy-,(aR)
Benzenemethanol, a-[(1S)-1-aminoethyl]-4-hydroxy-,(aR)![2(1H)-Quinolinone,5-[(1R)-2-amino-1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]ethyl]-8-hydroxy 2(1H)-Quinolinone,5-[(1R)-2-amino-1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]ethyl]-8-hydroxy](/data/attachment/201706/07/e0e9b5769a45af836d70be4140043125.gif.thumb.jpg) 2(1H)-Quinolinone,5-[(1R)-2-amino-1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]ethyl]-8-hydroxy
2(1H)-Quinolinone,5-[(1R)-2-amino-1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]ethyl]-8-hydroxy![2-{[3-(4-Fluorophenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrim idin-2-yl]sulfanyl}-N-(6-methyl-1,3-benzothiazol-2-yl)acetamide 2-{[3-(4-Fluorophenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrim idin-2-yl]sulfanyl}-N-(6-methyl-1,3-benzothiazol-2-yl)acetamide](/data/attachment/201706/07/5754ee36bdfbf4148f45632422f563b9.jpg) 2-{[3-(4-Fluorophenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrim idin-2-yl]sulfanyl}-N-(6-methyl-1,3-benzothiazol-2-yl)acetamide
2-{[3-(4-Fluorophenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrim idin-2-yl]sulfanyl}-N-(6-methyl-1,3-benzothiazol-2-yl)acetamide![2-((3-(2-methoxyphenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrimidin-2-yl)thio)-N-(6-methylbenzo[d]thiazol-2-yl)acetamide 2-((3-(2-methoxyphenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrimidin-2-yl)thio)-N-(6-methylbenzo[d]thiazol-2-yl)acetamide](/data/attachment/201706/08/47a8b3c98aef0b9ba378c4b7c6cef435.jpg) 2-((3-(2-methoxyphenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrimidin-2-yl)thio)-N-(6-methylbenzo[d]thiazol-2-yl)acetamide
2-((3-(2-methoxyphenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrimidin-2-yl)thio)-N-(6-methylbenzo[d]thiazol-2-yl)acetamide![N-(6-Methyl-2-benzothiazolyl)-2-[(3,4,6,7-tetrahydro-4-oxo-3-phenylthieno[3,2-d]pyrimidin-2-yl)thio]-acetamide N-(6-Methyl-2-benzothiazolyl)-2-[(3,4,6,7-tetrahydro-4-oxo-3-phenylthieno[3,2-d]pyrimidin-2-yl)thio]-acetamide](/data/attachment/201706/08/beda6f8f4655aa74d3646cfc7621fb20.jpg) N-(6-Methyl-2-benzothiazolyl)-2-[(3,4,6,7-tetrahydro-4-oxo-3-phenylthieno[3,2-d]pyrimidin-2-yl)thio]-acetamide
N-(6-Methyl-2-benzothiazolyl)-2-[(3,4,6,7-tetrahydro-4-oxo-3-phenylthieno[3,2-d]pyrimidin-2-yl)thio]-acetamide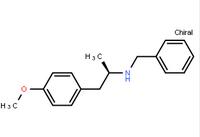 (R)-(-)-1-(4-methoxyphenyl)-2-benzylaminopropane
(R)-(-)-1-(4-methoxyphenyl)-2-benzylaminopropane![4(3H)-Quinazolinone,7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl] 4(3H)-Quinazolinone,7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]](/data/attachment/201706/09/b600ffca12695094db2c5f6045cb6685.jpg) 4(3H)-Quinazolinone,7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]
4(3H)-Quinazolinone,7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]![9-OXO-1,2,3,9-TETRAHYDRO-PYRROLO[2,1-B]QUINAZOLINE-6-CARBOXYLIC ACID 9-OXO-1,2,3,9-TETRAHYDRO-PYRROLO[2,1-B]QUINAZOLINE-6-CARBOXYLIC ACID](/data/attachment/201706/09/d6b395bbfb23e628be7d536d9cc2b512.gif) 9-OXO-1,2,3,9-TETRAHYDRO-PYRROLO[2,1-B]QUINAZOLINE-6-CARBOXYLIC ACID
9-OXO-1,2,3,9-TETRAHYDRO-PYRROLO[2,1-B]QUINAZOLINE-6-CARBOXYLIC ACID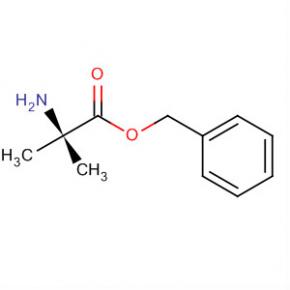 Alanine, 2-methyl-, phenylmethyl ester
Alanine, 2-methyl-, phenylmethyl ester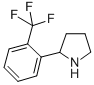 2-(2-TRIFLUOROMETHYL-PHENYL)-PYRROLIDINE
2-(2-TRIFLUOROMETHYL-PHENYL)-PYRROLIDINE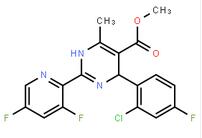 (-)-4(R)-(2-Chloro-4-fluorophenyl)-2-(3,5-difluoropyridin-2-yl)-6-methyl-1,4-dihydropyrimidine-5-carboxylic acid methyl ester
(-)-4(R)-(2-Chloro-4-fluorophenyl)-2-(3,5-difluoropyridin-2-yl)-6-methyl-1,4-dihydropyrimidine-5-carboxylic acid methyl ester![2-Butenoic acid, 2-hydroxy-4-[5-(1-Methylethyl)-2,4-bis(phenylMethoxy)phenyl]-4-oxo-, ethyl ester 2-Butenoic acid, 2-hydroxy-4-[5-(1-Methylethyl)-2,4-bis(phenylMethoxy)phenyl]-4-oxo-, ethyl ester](/data/attachment/201706/09/11c6e17ba89840528c5461ae5350df33.gif) 2-Butenoic acid, 2-hydroxy-4-[5-(1-Methylethyl)-2,4-bis(phenylMethoxy)phenyl]-4-oxo-, ethyl ester
2-Butenoic acid, 2-hydroxy-4-[5-(1-Methylethyl)-2,4-bis(phenylMethoxy)phenyl]-4-oxo-, ethyl estermethanone [4-amino-2-(ethylsulfanyl)pyrimidin-5-yl](2,3-difluoro-6-methoxyphenyl)methanone](/data/attachment/201706/09/ca947be16560699c92609cd96b352c02.png.thumb.jpg) [4-amino-2-(ethylsulfanyl)pyrimidin-5-yl](2,3-difluoro-6-methoxyphenyl)methanone
[4-amino-2-(ethylsulfanyl)pyrimidin-5-yl](2,3-difluoro-6-methoxyphenyl)methanone![Benzenesulfonamide, 2-[(5-bromo-2-chloro-4-pyrimidinyl)amino]-N-methyl Benzenesulfonamide, 2-[(5-bromo-2-chloro-4-pyrimidinyl)amino]-N-methyl](/data/attachment/201706/10/ce0d621896c03bdb67e3b184103e84ff.png.thumb.jpg) Benzenesulfonamide, 2-[(5-bromo-2-chloro-4-pyrimidinyl)amino]-N-methyl
Benzenesulfonamide, 2-[(5-bromo-2-chloro-4-pyrimidinyl)amino]-N-methyl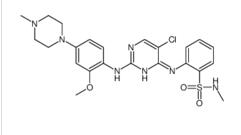 ALK inhibitor 2
ALK inhibitor 2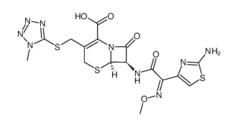 Cefmenoxime hydrochloride
Cefmenoxime hydrochloride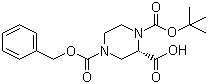 (S)-N-1-Boc-N-4-Cbz-2-piperazinecarboxylic acid
(S)-N-1-Boc-N-4-Cbz-2-piperazinecarboxylic acid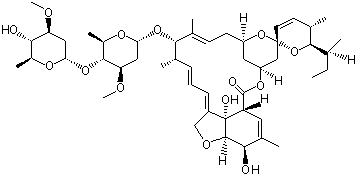 Avermectin
Avermectin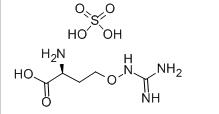 L-CANAVANINE SULFATE
L-CANAVANINE SULFATE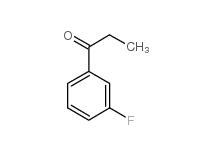 3-Fluoropropiophenone 455-67-4
3-Fluoropropiophenone 455-67-4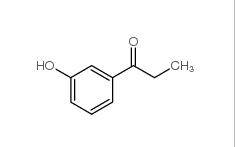 3-Hydroxypropiophenone 13103-80-5
3-Hydroxypropiophenone 13103-80-5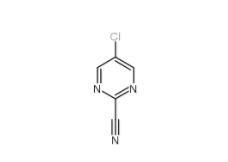 2-Cyano-5-chloropyrimidine 38275-56-8
2-Cyano-5-chloropyrimidine 38275-56-8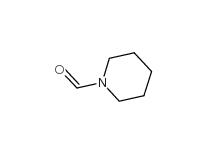 N-Formylpiperidine 2591-86-8
N-Formylpiperidine 2591-86-8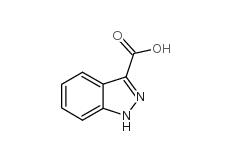 Indazole-3-carboxylic acid 4498-67-3
Indazole-3-carboxylic acid 4498-67-3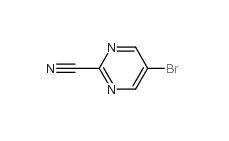 5-Bromo-2-cyanopyrimidine 38275-57-9
5-Bromo-2-cyanopyrimidine 38275-57-9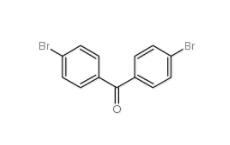 4,4-Dibromobenzophenone 3988-03-2
4,4-Dibromobenzophenone 3988-03-2![1,2,3,4-Tetrahydro-benzo[b]azepin-5-one 1127-74-8 1,2,3,4-Tetrahydro-benzo[b]azepin-5-one 1127-74-8](/data/attachment/201901/28/b183df1e648eca4396ad0d319a1254bc.jpg) 1,2,3,4-Tetrahydro-benzo[b]azepin-5-one 1127-74-8
1,2,3,4-Tetrahydro-benzo[b]azepin-5-one 1127-74-8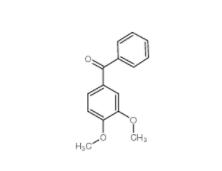 4038-14-6,(3,4-dimethoxyphenyl)-phenylmethanone 4038-14-6
4038-14-6,(3,4-dimethoxyphenyl)-phenylmethanone 4038-14-6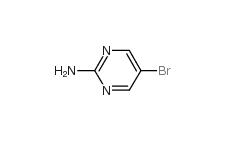 2-Amino-5-bromopyrimidine 7752-82-1
2-Amino-5-bromopyrimidine 7752-82-1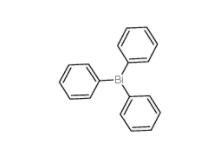 Triphenylbismuth 603-33-8
Triphenylbismuth 603-33-8![3-Ethynylimidazo[1,2-a]pyridine 943320-53-4 3-Ethynylimidazo[1,2-a]pyridine 943320-53-4](/data/attachment/201901/28/d6294d1dabcee85ee04792b0c0e255c0.jpg) 3-Ethynylimidazo[1,2-a]pyridine 943320-53-4
3-Ethynylimidazo[1,2-a]pyridine 943320-53-4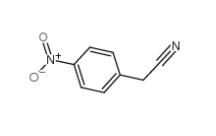 4-Nitrophenylacetonitrile 555-21-5
4-Nitrophenylacetonitrile 555-21-5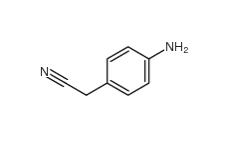 2-(4-aminophenyl)acetonitrile 3544-25-0
2-(4-aminophenyl)acetonitrile 3544-25-0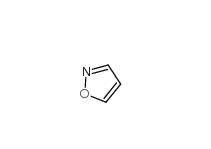 isoxazole 288-14-2
isoxazole 288-14-2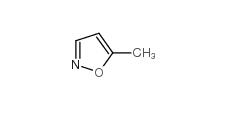 5-Methylisoxazole 5765-44-6
5-Methylisoxazole 5765-44-6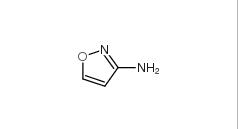 3-Aminoisoxazole 1750-42-1
3-Aminoisoxazole 1750-42-1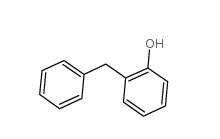 2-Hydroxydiphenylmethane 28994-41-4
2-Hydroxydiphenylmethane 28994-41-4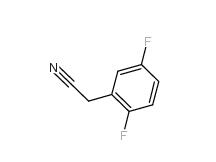 2,5-Difluorobenzyl Cyanide 69584-87-8
2,5-Difluorobenzyl Cyanide 69584-87-8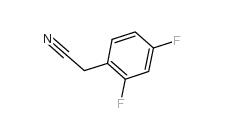 2,4-Difluorophenylacetonitrile 656-35-9
2,4-Difluorophenylacetonitrile 656-35-9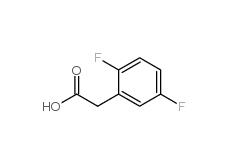 2,5-Difluorophenylacetic acid 85068-27-5
2,5-Difluorophenylacetic acid 85068-27-5 2,4-Difluorophenylacetic acid 81228-09-3
2,4-Difluorophenylacetic acid 81228-09-3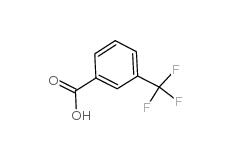 3-trifluoromethylbenzoic acid 454-92-2
3-trifluoromethylbenzoic acid 454-92-2![2-[3-(trifluoromethyl)phenyl]acetonitrile 2338-76-3 2-[3-(trifluoromethyl)phenyl]acetonitrile 2338-76-3](/data/attachment/201901/29/22b99245cb0bbcd2d86f238725d9fb9d.jpg) 2-[3-(trifluoromethyl)phenyl]acetonitrile 2338-76-3
2-[3-(trifluoromethyl)phenyl]acetonitrile 2338-76-3![2-[3-(trifluoromethyl)phenyl]acetic acid 351-35-9 2-[3-(trifluoromethyl)phenyl]acetic acid 351-35-9](/data/attachment/201901/29/7dbc74a276a4c124b9460222442fd80f.jpg) 2-[3-(trifluoromethyl)phenyl]acetic acid 351-35-9
2-[3-(trifluoromethyl)phenyl]acetic acid 351-35-9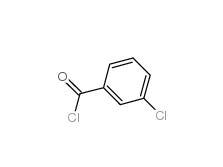 3-Chlorobenzoyl chloride 618-46-2
3-Chlorobenzoyl chloride 618-46-2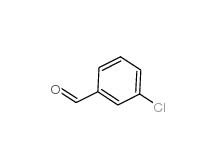 3-Chlorobenzaldehyde 587-04-2
3-Chlorobenzaldehyde 587-04-2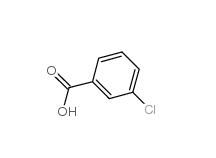 3-chlorobenzoic acid 535-80-8
3-chlorobenzoic acid 535-80-8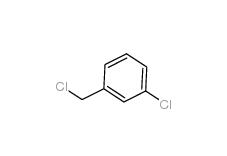 3-Chlorobenzyl chloride 620-20-2
3-Chlorobenzyl chloride 620-20-2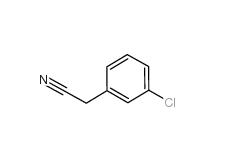 3-Chlorobenzyl cyanide 1529-41-5
3-Chlorobenzyl cyanide 1529-41-5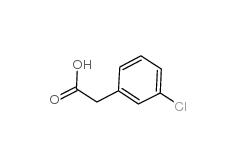 2-(3-chlorophenyl)acetic acid 1878-65-5
2-(3-chlorophenyl)acetic acid 1878-65-5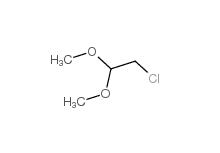 Dimethylchloroacetal 97-97-2
Dimethylchloroacetal 97-97-2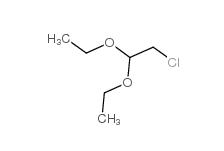 Chloroacetaldehyde diethyl acetal 621-62-5
Chloroacetaldehyde diethyl acetal 621-62-5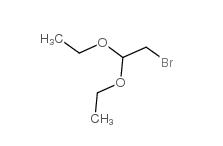 2-bromo-1,1-diethoxyethane 2032-35-1
2-bromo-1,1-diethoxyethane 2032-35-1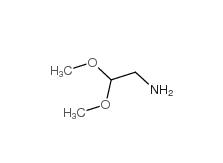 2,2-dimethoxyethanamine 22483-09-6
2,2-dimethoxyethanamine 22483-09-6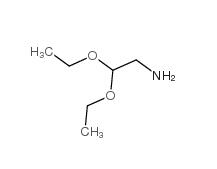 2,2-Diethoxyethylamine 645-36-3
2,2-Diethoxyethylamine 645-36-3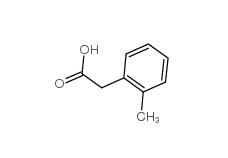 2-Methylphenylacetic acid 644-36-0
2-Methylphenylacetic acid 644-36-0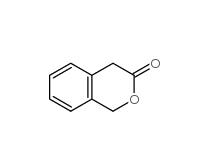 3-Isochromanone 4385-35-7
3-Isochromanone 4385-35-7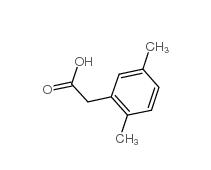 2,5-Dimethylphenylacetic acid 13612-34-5
2,5-Dimethylphenylacetic acid 13612-34-5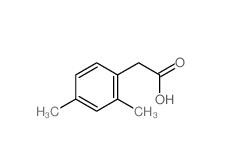 2,4-Dimethylphenylacetic Acid 6331-04-0
2,4-Dimethylphenylacetic Acid 6331-04-0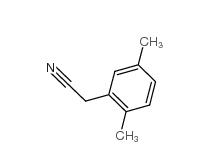 2,5-Dimethylphenylacetonitrile 16213-85-7
2,5-Dimethylphenylacetonitrile 16213-85-7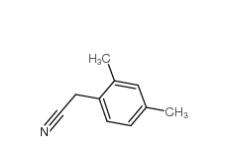 2,4-Dimethylphenylacetonitrile 68429-53-8
2,4-Dimethylphenylacetonitrile 68429-53-8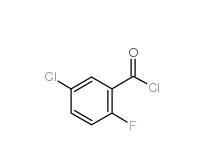 5-CHLORO-2-FLUOROBENZOYL CHLORIDE 394-29-6
5-CHLORO-2-FLUOROBENZOYL CHLORIDE 394-29-6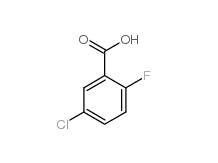 5-Chloro-2-fluorobenzoic acid 394-30-9
5-Chloro-2-fluorobenzoic acid 394-30-9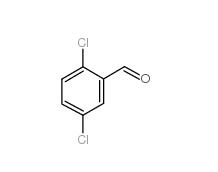 2,5-Dichlorobenzaldehyde 6361-23-5
2,5-Dichlorobenzaldehyde 6361-23-5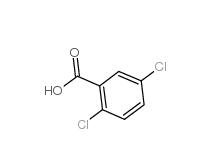 2,5-Dichlorobenzoic acid 50-79-3
2,5-Dichlorobenzoic acid 50-79-3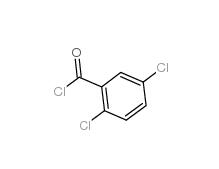 2,5-DICHLOROBENZOYL CHLORIDE 2905-61-5
2,5-DICHLOROBENZOYL CHLORIDE 2905-61-5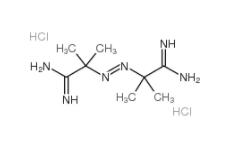 2,2-Azobis(2-methylpropionamidine) dihydrochloride 2997-92-4
2,2-Azobis(2-methylpropionamidine) dihydrochloride 2997-92-4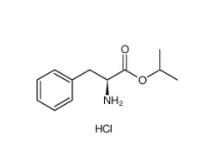 L-Phenylalanine, 1-methylethyl ester, hydrochloride 95585-78-7
L-Phenylalanine, 1-methylethyl ester, hydrochloride 95585-78-7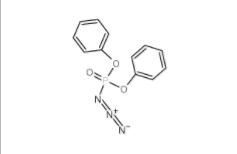 Diphenylphosphoryl azide 26386-88-9
Diphenylphosphoryl azide 26386-88-9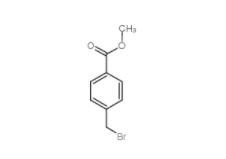 Methyl 4-(bromomethyl)benzoate 2417-72-3
Methyl 4-(bromomethyl)benzoate 2417-72-3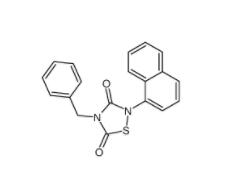 Tideglusib 865854-05-3
Tideglusib 865854-05-3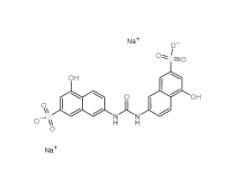 Disodium 7,7-(carbonyldiimino)bis(4-hydroxynaphthalene-2-sulphonate) 20324-87-2
Disodium 7,7-(carbonyldiimino)bis(4-hydroxynaphthalene-2-sulphonate) 20324-87-2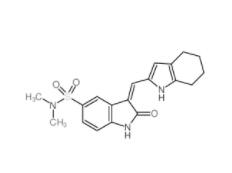 SU 6656 330161-87-0
SU 6656 330161-87-0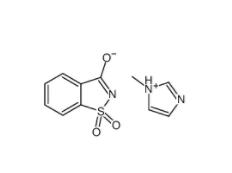 Saccharin 1-methylimidazole 482333-74-4
Saccharin 1-methylimidazole 482333-74-4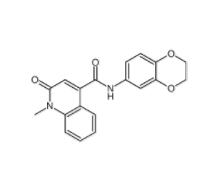 CeMMEC13 1790895-25-8
CeMMEC13 1790895-25-8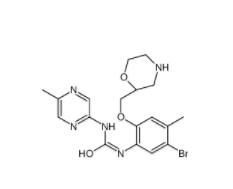 Rabusertib 911222-45-2
Rabusertib 911222-45-2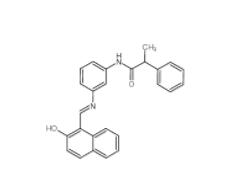 Salermide 1105698-15-4
Salermide 1105698-15-4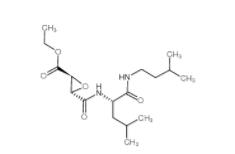 EST 88321-09-9
EST 88321-09-9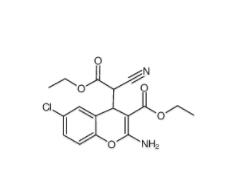 SC79 305834-79-1
SC79 305834-79-1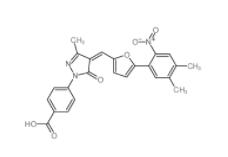 C646 328968-36-1
C646 328968-36-1 1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea 182498-32-4
1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea 182498-32-4 Dp44mT 152095-12-0
Dp44mT 152095-12-0 Deguelin 522-17-8
Deguelin 522-17-8 PD168393 194423-15-9
PD168393 194423-15-9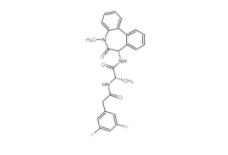 YO01027 209984-56-5
YO01027 209984-56-5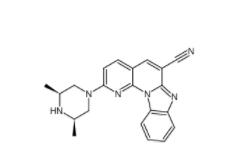 DC10539 1822358-25-7
DC10539 1822358-25-7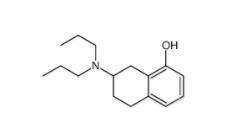 8-OH-DPAT 78950-78-4
8-OH-DPAT 78950-78-4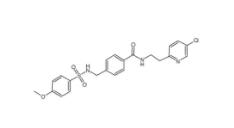 YU238259 1943733-16-1
YU238259 1943733-16-1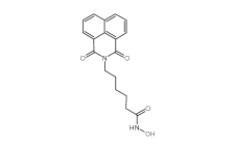 Scriptaid 287383-59-9
Scriptaid 287383-59-9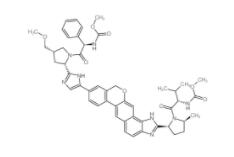 Velpatasvir 1377049-84-7
Velpatasvir 1377049-84-7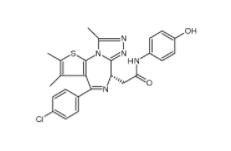 OTX015 202590-98-5
OTX015 202590-98-5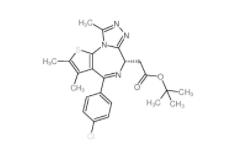 (+)-JQ-1 1268524-70-4
(+)-JQ-1 1268524-70-4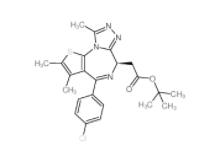 (-)-JQ-1 1268524-71-5
(-)-JQ-1 1268524-71-5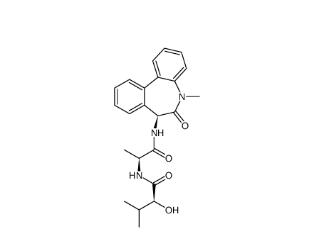 LY 900009 209984-68-9
LY 900009 209984-68-9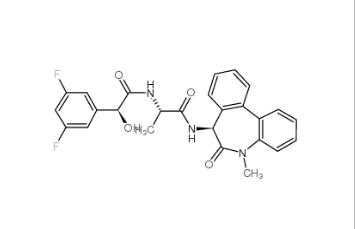 LY-411575 209984-57-6
LY-411575 209984-57-6![(4-nitrophenyl) 4-[bis(1,3-benzodioxol-5-yl)-hydroxymethyl]piperidine-1-carboxylate 1101854-58-3 (4-nitrophenyl) 4-[bis(1,3-benzodioxol-5-yl)-hydroxymethyl]piperidine-1-carboxylate 1101854-58-3](/data/attachment/201903/22/9e81dae7e0bdec56ac6052b1872d9626.jpg) (4-nitrophenyl) 4-[bis(1,3-benzodioxol-5-yl)-hydroxymethyl]piperidine-1-carboxylate 1101854-58-3
(4-nitrophenyl) 4-[bis(1,3-benzodioxol-5-yl)-hydroxymethyl]piperidine-1-carboxylate 1101854-58-3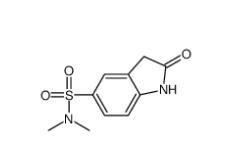 N,N-dimethyl-2-oxo-1,3-dihydroindole-5-sulfonamide 170565-89-6
N,N-dimethyl-2-oxo-1,3-dihydroindole-5-sulfonamide 170565-89-6![5,6,11,12-tetrahydrodibenzo[1,2-b:1,2-g][8]annulene 1460-59-9 5,6,11,12-tetrahydrodibenzo[1,2-b:1,2-g][8]annulene 1460-59-9](/data/attachment/201903/23/50930df6d55412ac8f4da0724b497aaf.jpg) 5,6,11,12-tetrahydrodibenzo[1,2-b:1,2-g][8]annulene 1460-59-9
5,6,11,12-tetrahydrodibenzo[1,2-b:1,2-g][8]annulene 1460-59-9 Tetraphenylmethane 630-76-2
Tetraphenylmethane 630-76-2![2-chloro-6-[(2R)-2-hydroxy-3-[(2-methyl-1-naphthalen-2-ylpropan-2-yl)amino]propoxy]benzonitrile 284035-33-2 2-chloro-6-[(2R)-2-hydroxy-3-[(2-methyl-1-naphthalen-2-ylpropan-2-yl)amino]propoxy]benzonitrile 284035-33-2](/data/attachment/201903/23/7e63bafe6c4b7e146e00c57dfca99672.jpg) 2-chloro-6-[(2R)-2-hydroxy-3-[(2-methyl-1-naphthalen-2-ylpropan-2-yl)amino]propoxy]benzonitrile 284035-33-2
2-chloro-6-[(2R)-2-hydroxy-3-[(2-methyl-1-naphthalen-2-ylpropan-2-yl)amino]propoxy]benzonitrile 284035-33-2 1616380-54-1
1616380-54-1![N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-methyl-1-oxo-1,2-dihydroisoquinoline-4-carboxamide 440662-09-9 N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-methyl-1-oxo-1,2-dihydroisoquinoline-4-carboxamide 440662-09-9](/data/attachment/201903/23/e26baac537719657acd9f1f55568401d.jpg) N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-methyl-1-oxo-1,2-dihydroisoquinoline-4-carboxamide 440662-09-9
N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-methyl-1-oxo-1,2-dihydroisoquinoline-4-carboxamide 440662-09-9![N-[2-[2-(4-chlorophenyl)-1,3-thiazol-4-yl]ethyl]butanamide 932986-18-0 N-[2-[2-(4-chlorophenyl)-1,3-thiazol-4-yl]ethyl]butanamide 932986-18-0](/data/attachment/201903/23/7d2bbd100c8322ae16168937617e1bb2.jpg) N-[2-[2-(4-chlorophenyl)-1,3-thiazol-4-yl]ethyl]butanamide 932986-18-0
N-[2-[2-(4-chlorophenyl)-1,3-thiazol-4-yl]ethyl]butanamide 932986-18-0 5-(5-(2-(3-aminopropoxy)-6-methoxyphenyl)-1H-pyrazol-3-ylamino)pyrazine-2-carbonitrile 1234015-52-1
5-(5-(2-(3-aminopropoxy)-6-methoxyphenyl)-1H-pyrazol-3-ylamino)pyrazine-2-carbonitrile 1234015-52-1![3-Methyl-3-[(1E)-2-phenylethenyl]-3,2:5,2:5,3-quaterpyridine 1651890-44-6 3-Methyl-3-[(1E)-2-phenylethenyl]-3,2:5,2:5,3-quaterpyridine 1651890-44-6](/data/attachment/201903/23/07bf6fd99e81033df0c83039ccdde036.jpg) 3-Methyl-3-[(1E)-2-phenylethenyl]-3,2:5,2:5,3-quaterpyridine 1651890-44-6
3-Methyl-3-[(1E)-2-phenylethenyl]-3,2:5,2:5,3-quaterpyridine 1651890-44-6![3-[4-(Dimethylamino)-3-biphenylyl]-1,1-dimethylure 1469924-27-3 3-[4-(Dimethylamino)-3-biphenylyl]-1,1-dimethylure 1469924-27-3](/data/attachment/201903/23/f69ad7342d131146640e0c88f73e9a25.jpg) 3-[4-(Dimethylamino)-3-biphenylyl]-1,1-dimethylure 1469924-27-3
3-[4-(Dimethylamino)-3-biphenylyl]-1,1-dimethylure 1469924-27-3 8-Methoxy-N,N-dipropyl-1,2,3,4-tetrahydro-2-naphthalenamine 3897-94-7
8-Methoxy-N,N-dipropyl-1,2,3,4-tetrahydro-2-naphthalenamine 3897-94-7![4-{4-[(4-{[3-(Acryloylamino)phenyl]amino}-5-fluoro-2-pyrimidinyl) amino]phenoxy}-N-methyl-2-pyridinecarboxamide 1202759-32-7 4-{4-[(4-{[3-(Acryloylamino)phenyl]amino}-5-fluoro-2-pyrimidinyl) amino]phenoxy}-N-methyl-2-pyridinecarboxamide 1202759-32-7](/data/attachment/201903/23/bb4110673d0676f81860d708092eb660.jpg) 4-{4-[(4-{[3-(Acryloylamino)phenyl]amino}-5-fluoro-2-pyrimidinyl) amino]phenoxy}-N-methyl-2-pyridinecarboxamide 1202759-32-7
4-{4-[(4-{[3-(Acryloylamino)phenyl]amino}-5-fluoro-2-pyrimidinyl) amino]phenoxy}-N-methyl-2-pyridinecarboxamide 1202759-32-7 1,4-Dihydro-2,6-dimethyl-3-nitro-4-(2-trifluoromethylphenyl)-pyridine-5-carboxylic acid methyl ester 71145-03-4
1,4-Dihydro-2,6-dimethyl-3-nitro-4-(2-trifluoromethylphenyl)-pyridine-5-carboxylic acid methyl ester 71145-03-4![2-[[3-[[2-(dimethylamino)phenyl]methyl]-2-pyridin-4-yl-1,3-diazinan-1-yl]methyl]-N,N-dimethylaniline 500579-04-4 2-[[3-[[2-(dimethylamino)phenyl]methyl]-2-pyridin-4-yl-1,3-diazinan-1-yl]methyl]-N,N-dimethylaniline 500579-04-4](/data/attachment/201903/23/b396a2326dddb511aae497b01fbd4c77.jpg) 2-[[3-[[2-(dimethylamino)phenyl]methyl]-2-pyridin-4-yl-1,3-diazinan-1-yl]methyl]-N,N-dimethylaniline 500579-04-4
2-[[3-[[2-(dimethylamino)phenyl]methyl]-2-pyridin-4-yl-1,3-diazinan-1-yl]methyl]-N,N-dimethylaniline 500579-04-4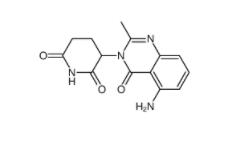 CC-122 1015474-32-4
CC-122 1015474-32-4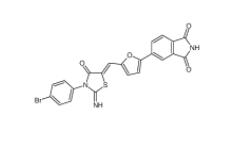 Bioymifi 1420071-30-2
Bioymifi 1420071-30-2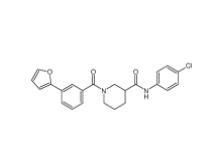 N-(4-chlorophenyl)-1-(3-(furan-2-yl)benzoyl)piperidine-3-carboxamide 1443437-74-8
N-(4-chlorophenyl)-1-(3-(furan-2-yl)benzoyl)piperidine-3-carboxamide 1443437-74-8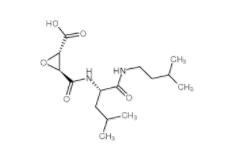 E-64C 76684-89-4
E-64C 76684-89-4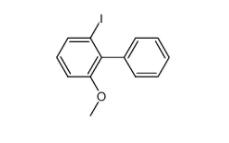 2-iodo-6-methoxybiphenyl 84253-78-1
2-iodo-6-methoxybiphenyl 84253-78-1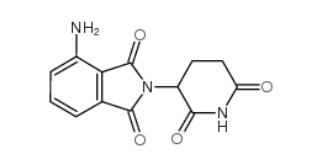 pomalidomide 19171-19-8
pomalidomide 19171-19-8 4EP-Directory listing
4EP-Directory listing Stearoylbenzoylmethane 58446-52-9
Stearoylbenzoylmethane 58446-52-9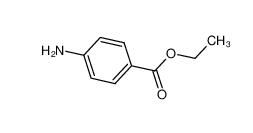 benzocaine 94-09-7
benzocaine 94-09-7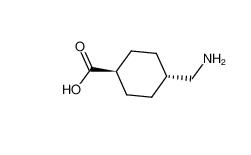 tranexamic acid 1197-18-8
tranexamic acid 1197-18-8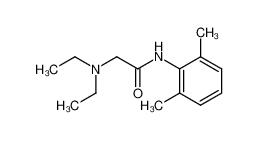 lidocaine 137-58-6
lidocaine 137-58-6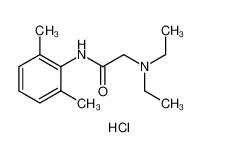 lidocaine hydrochloride 73-78-9
lidocaine hydrochloride 73-78-9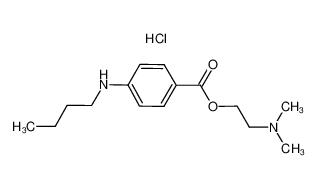 Tetracaine hydrochloride 136-47-0
Tetracaine hydrochloride 136-47-0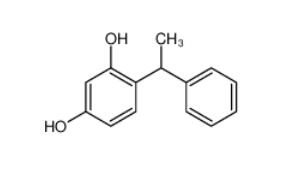 4-(1-phenylethyl)benzene-1,3-diol 85-27-8
4-(1-phenylethyl)benzene-1,3-diol 85-27-8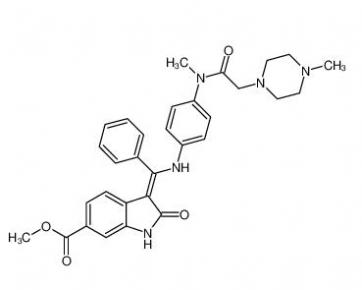 Nintedanib 656247-17-5
Nintedanib 656247-17-5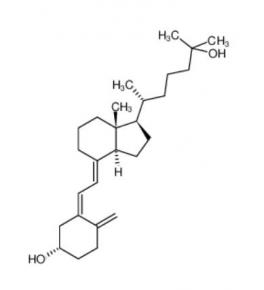 calcidiol 19356-17-3
calcidiol 19356-17-3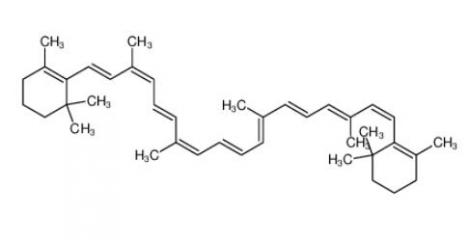 β-carotene 7235-40-7
β-carotene 7235-40-7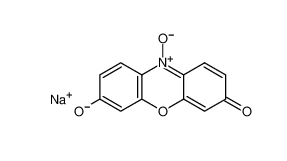 Resazurin sodium salt 62758-13-8
Resazurin sodium salt 62758-13-8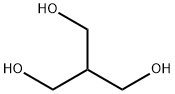 4704-94-3 2-(HYDROXYMETHYL)-1,3-PROPANEDIOL
4704-94-3 2-(HYDROXYMETHYL)-1,3-PROPANEDIOL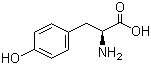 L-Tyrosine 60-18-4
L-Tyrosine 60-18-4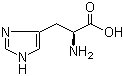 L-Histidine 71-00-1
L-Histidine 71-00-1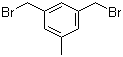 3,5-Bis(bromomethyl)toluene 19294-04-3
3,5-Bis(bromomethyl)toluene 19294-04-3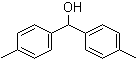 Bis(4-methylphenyl)methanol 885-77-8
Bis(4-methylphenyl)methanol 885-77-8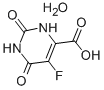 5-Fluoroorotic Acid Hydrate 207291-81-4
5-Fluoroorotic Acid Hydrate 207291-81-4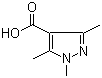 1,3,5-Trimethyl-1H-pyrazole-4-carboxylic acid 1125-29-7
1,3,5-Trimethyl-1H-pyrazole-4-carboxylic acid 1125-29-7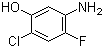 5-Amino-2-chloro-4-fluorophenol 84478-72-8
5-Amino-2-chloro-4-fluorophenol 84478-72-8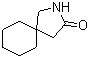 Gabapentin-lactam 64744-50-9
Gabapentin-lactam 64744-50-9 1EP-Directory listing
1EP-Directory listing![[2-(aminocarbonyl)phenyl]acetic acid 23362-56-3 [2-(aminocarbonyl)phenyl]acetic acid 23362-56-3](/data/attachment/202211/10/9756043560e11c17cf958f3ed54d541a.png.thumb.jpg) [2-(aminocarbonyl)phenyl]acetic acid 23362-56-3
[2-(aminocarbonyl)phenyl]acetic acid 23362-56-3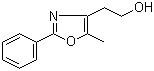 2-(5-Methyl-2-phenyl-1,3-oxazol-4-yl)ethan-1-ol 103788-65-4
2-(5-Methyl-2-phenyl-1,3-oxazol-4-yl)ethan-1-ol 103788-65-4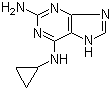 2-Amino-6-cyclopropylamino-9H-purine 120503-69-7
2-Amino-6-cyclopropylamino-9H-purine 120503-69-7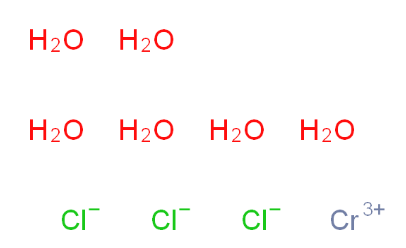 Chromic chloride hexahydrate 10060-12-5
Chromic chloride hexahydrate 10060-12-5 2EP-Directory listing 2
2EP-Directory listing 2 3EP-Directory listing 3
3EP-Directory listing 3





