Product Name: Regorafenib
Synonyms: Regorafenib;BAY 73-4506;4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide;BAY 73-4506(Regorafenib);4-(4-(3-(4-chloro-3-(trifluoroMethyl)phenyl)ureido)-3-fluorophenoxy)-N-MethylpicolinaMide;4-[4-({[4-chioro-3-(trifluoroMethyl)phenyl]carbaMoyl}aMino)-3-fluorophenoxy]-pyridine-2-carboxylic acid MethylaMide;REGORAFENIB BAY 73-4506 BAYER HEALTHCARE (SEE ALSO COLORECTAL, LUNG, STOMACH) PHASE II(REGORAFENIB) PHARMACEUTICALS (888) 842-2937WAYNE, NJ;Regorafenib (BAY 73-4506)
CAS: 755037-03-7
MF: C21H15ClF4N4O3
MW: 482.82
EINECS: 815-051-1
Product Categories: Inhibitors;API;Amines;Aromatics;Heterocycles;Anti-cancer&immunity;Intermediates & Fine Chemicals;Pharmaceuticals
Mol File: 755037-03-7.mol
Regorafenib Structure
Regorafenib Chemical Properties
Melting point 206.0 to 210.0 °C
Boiling point 513.4±50.0 °C(Predicted)
density 1.491±0.06 g/cm3(Predicted)
form White powder.
pka 12.04±0.70(Predicted)
CAS DataBase Reference 755037-03-7
Safety Information
HS Code 29242990
MSDS Information
Regorafenib Usage And Synthesis
Side effects
Regorafenib is being approved with a Boxed Warning alerting patients and health care professionals that severe and fatal liver toxicity occurred in patients treated with regorafenib during clinical studies. Serious side effects, which occurred in less than one percent of patients, were liver damage, severe bleeding, blistering and peeling of skin, very high blood pressures requiring emergency treatment, heart attacks and perforations (holes) in the intestines. The most common side effects reported in patients treated with regorafenib include weakness or fatigue, loss of appetite, hand-foot syndrome (also called palmar-plantar erythrodysesthesia), diarrhoea, mouth sores (mucositis), weight loss, infection, high blood pressure, and changes in voice volume or quality (dysphonia).
Small Molecule Inhibitor
Regorafenib (BAY 73-4506, Stivarga ) is a new oral small molecule multi-kinases inhibitor. It can inhibit the target kinases associated with angiogenesis and tumorigenesis. The pathway influenced by regorafenib and the biomarkers for monitoring the efficacy of regorafenib become hot spots. Because of its wide spectrum kinase inhibitory activity, the utilization of regorafenib in many clinical indications are also carried out extensively. Since regorafenib is approved with the box warning, its side effects can not be ignored.
FDA Approve
Regorafenib (BAY73-4506) is a new type of multikinase inhibitor developed by Bayer, and is the first small molecule kinase inhibitor approved by the U.S. FDA on September 27, 2012 used for fast track colorectal cancer that develops and metastases after conventional treatment.
Regorafenib achieves good results in some patients with rectal cancer that are resistant to traditional chemotherapy, but not all rectal cancers are sensitive to it. Therefore, the pathway influenced by regorafenib and the biomarkers for monitoring the efficacy of regorafenib become hot spots.
Description In September 2012, theUSFDAapproved regorafenib for the treatment of patients with metastatic colorectal cancer (CRC), especially those for whom standard therapies have failed, including fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, ananti-EGFRtherapy. Regorafenib is a multikinase inhibitor with potent inhibitory activity versus VEGFRs and PDFRs. Both of these classes of receptors are expressed on tumor cells and affect proliferation and angiogenesis. Regorafenib inhibited growth in murine xenograft models for colon, breast, renal, lung, melanoma, pancreatic, and ovarian tumors when dosed at 10–30 mg/kg. Regorafenib is a fluorinated analog of sorafenib, a multikinase inhibitor co-marketed by Bayer and Onyx for the treatment of kidney and liver cancer. The synthesis of regorafenib is accomplished in two steps from commercially available starting materials. 4-Aminophenol is coupled to 4-chloro-N-methyl- 2-pyridinecarboxamide to give 4-(2-(N-methylcarbamoyl)-4-pyridyloxy)aniline. Subsequent treatment with 4-chloro-3-(trifluoromethyl)phenyl isocycanate affords the urea, regorafenib.
Description Regorafenib is an orally bioavailable multi-kinase inhibitor with anticancer activity. It inhibits RET, C-RAF, VEGFR2, c-Kit, VEGFR1, and PDGFRβ with IC50 values of 1.5, 2.5, 4.2, 7, 13, and 22 nM, respectively. Regorafenib also inhibits B-RAF, VEGFR3, FGFR, and Tie2 (IC50s = 28, 46, 202, and 311 nM, respectivey) as well as other kinases. In vivo, regorafenib (10 mg/kg) reduces tumor size in the MDA-MB-231 breast and 786-O renal cancer mouse xenograft models. It also reduces tumor microvessel area and inhibits tumor growth in a panel of mouse xenograft models. Formulations containing regorafenib have been used in the treatment of advanced gastrointestinal stromal tumors and metastatic colorectal cancer.
Originator Bayer (Germany)
Uses It inhibits PDGFR tyrosine kinase with IC50=83nM. It is useful for the treatment of inflammation and as an anti-proliferative agent.
Uses BAY 73-4506 (Regorafenib) is a multikinase inhibitor with IC50 of 17, 40 and 69 nM c-KIT, VEGFR2, B-Raf.
Uses Regorafenib (BAY 73-4506) is a multi-target inhibitor for VEGFR1, VEGFR2, VEGFR3, PDGFRβ, Kit, RET and Raf-1 with IC50 of 13 nM/4.2 nM/46 nM, 22 nM, 7 nM, 1.5 nM and 2.5 nM, respectively
Definition ChEBI: A pyridinecarboxamide obtained by condensation of 4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]pyridine-2-carboxylic acid with methylamine. Used for for the treatment of metastatic colorectal cancer in patients who have previ usly received chemotherapy, anti-EGFR or anti-VEGF therapy.
Brand name Stivarga
Clinical Use
Treatment of colorectal cancer and gastrointestinal stromal tumours
Treatment of hepatocellular carcinoma
Drug interactions Potentially hazardous interactions with other drugs
Analgesics: avoid with mefenamic acid.
Antibacterials: concentration reduced by rifampicin - avoid.
Anticoagulants: increased risk of bleeding with warfarin.
Antifungals: concentration increased by ketoconazole - avoid.
Antipsychotics: avoid with clozapine (increased risk of agranulocytosis).
Metabolism Regorafenib is metabolised by CYP3A4 and UGT1A9. The main circulating metabolites of regorafenib measured at steady-state in human plasma are M-2 (N-oxide) and M-5 (N-oxide and N-desmethyl), both of them having similar in vitro pharmacological activity and steady-state concentrations as regorafenib. M-2 and M-5 are highly protein bound (99.8% and 99.95%, respectively). Approximately 90% of the radioactive dose was recovered within 12 days after administration, with about 71% of the dose excreted in faeces (47% as parent compound, 24% as metabolites), and about 19% of the dose excreted in urine as glucuronides. Urinary excretion of glucuronides decreased below 10% under steady-state conditions. Parent compound found in faeces could be derived from intestinal degradation of glucuronides or reduction of metabolite M-2 (N-oxide), as well as unabsorbed regorafenib.
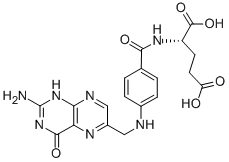 Folic acid,59-30-3
Folic acid,59-30-3
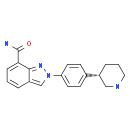 MK-4827 (HCl)
MK-4827 (HCl)
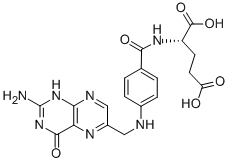 Folic acid
Folic acid
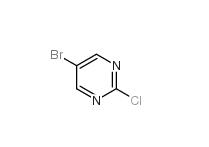 5-Bromo-2-chloropyrimidine 32779-36-5
5-Bromo-2-chloropyrimidine 32779-36-5
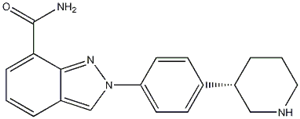 Niraparib
Niraparib
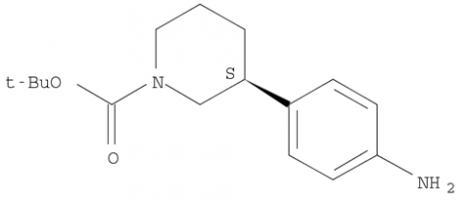 (R)-tert-butyl 3-(4-aminophenyl)piperidine-1-carboxylate
(R)-tert-butyl 3-(4-aminophenyl)piperidine-1-carboxylate
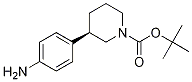 (R)-tert-butyl 3-(4-aMinophenyl)piperidine-1-carboxylate
(R)-tert-butyl 3-(4-aMinophenyl)piperidine-1-carboxylate
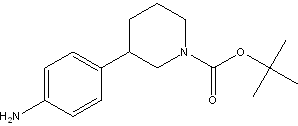 tert-butyl 3-(4-aminophenyl)piperidine-1-carboxylate
tert-butyl 3-(4-aminophenyl)piperidine-1-carboxylate
![(3S)-3-[4-[7-[[(1,1-Dimethylethyl)amino]carbonyl]-2H-indazol-2-yl]phenyl]-1-piperidinecarboxylic acid 1,1-dimethylethyl ester (3S)-3-[4-[7-[[(1,1-Dimethylethyl)amino]carbonyl]-2H-indazol-2-yl]phenyl]-1-piperidinecarboxylic acid 1,1-dimethylethyl ester](/data/attachment/201705/26/3446bd2b841689a5afc36447418dc476.png) (3S)-3-[4-[7-[[(1,1-Dimethylethyl)amino]carbonyl]-2H-indazol-2-yl]phenyl]-1-piperidinecarboxylic acid 1,1-dimethylethyl ester
(3S)-3-[4-[7-[[(1,1-Dimethylethyl)amino]carbonyl]-2H-indazol-2-yl]phenyl]-1-piperidinecarboxylic acid 1,1-dimethylethyl ester
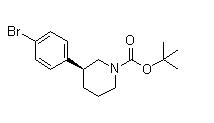 (3S)-3-(4-Bromophenyl)-1-piperidinecarboxylic acid 1,1-dimethylethyl ester
(3S)-3-(4-Bromophenyl)-1-piperidinecarboxylic acid 1,1-dimethylethyl ester
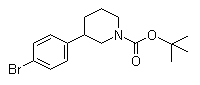 3-(4-Bromophenyl)piperidine-1-carboxylic acid tert-butyl ester
3-(4-Bromophenyl)piperidine-1-carboxylic acid tert-butyl ester
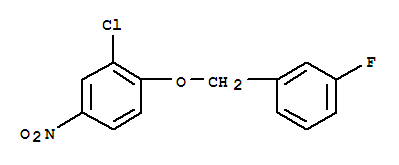 3-Chloro-4-(3-fluorobenzyloxy)nitrobenzene
3-Chloro-4-(3-fluorobenzyloxy)nitrobenzene
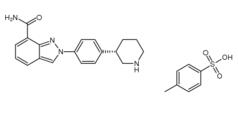 Niraparib p-toluenesulfonate
Niraparib p-toluenesulfonate
![N-(1,1-Dimethylethyl)-2-[4-(3S)-3-piperidinylphenyl]-2H-indazole-7-carboxamide N-(1,1-Dimethylethyl)-2-[4-(3S)-3-piperidinylphenyl]-2H-indazole-7-carboxamide](/data/attachment/201705/26/da41ae70b523a458db70333bd1059362.png) N-(1,1-Dimethylethyl)-2-[4-(3S)-3-piperidinylphenyl]-2H-indazole-7-carboxamide
N-(1,1-Dimethylethyl)-2-[4-(3S)-3-piperidinylphenyl]-2H-indazole-7-carboxamide
![2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide](/data/attachment/201705/26/d3114dd994f3dda3142cba7d326bcede.jpg) 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide
2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide
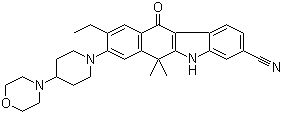 Alectinib
Alectinib
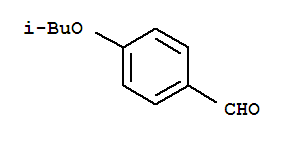 Benzaldehyde,4-(2-methylpropoxy)
Benzaldehyde,4-(2-methylpropoxy)
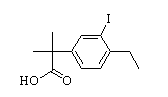 2-(4-ethyl-3-iodophenyl)-2-Methylpropanoic acid
2-(4-ethyl-3-iodophenyl)-2-Methylpropanoic acid
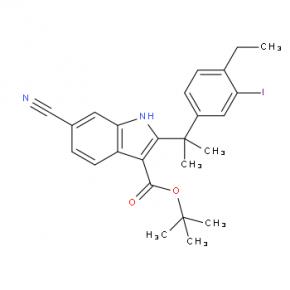 tert-butyl 6-cyano-2-(2-(4-ethyl-3-iodophenyl)propan-2-yl)-1H-indole-3-carboxylate
tert-butyl 6-cyano-2-(2-(4-ethyl-3-iodophenyl)propan-2-yl)-1H-indole-3-carboxylate
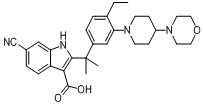 6-cyano-2-(2-(4-ethyl-3-(4-morpholinopiperidin-1-yl)phenyl)propan-2-yl)-1H-indole-3-carboxylic acid
6-cyano-2-(2-(4-ethyl-3-(4-morpholinopiperidin-1-yl)phenyl)propan-2-yl)-1H-indole-3-carboxylic acid
![9-ethyl-8-iodo-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile 9-ethyl-8-iodo-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile](/data/attachment/201705/28/e48e5d316800efe6192ebfdeec6cf28c.gif) 9-ethyl-8-iodo-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile
9-ethyl-8-iodo-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile
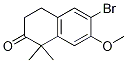 6-broMo-7-Methoxy-1,1-diMethyl-3,4-dihydronaphthalen-2(1H)-one
6-broMo-7-Methoxy-1,1-diMethyl-3,4-dihydronaphthalen-2(1H)-one
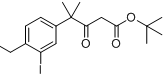 tert-butyl 4-(4-ethyl-3-iodophenyl)-4-methyl-3-oxopentanoate
tert-butyl 4-(4-ethyl-3-iodophenyl)-4-methyl-3-oxopentanoate
![9-broMo-8-hydroxy-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile 9-broMo-8-hydroxy-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile](/data/attachment/201705/28/fe98529212eb834b17a38f13138a35bf.png) 9-broMo-8-hydroxy-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile
9-broMo-8-hydroxy-6,6-diMethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile
![9-ethyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11a-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride 9-ethyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11a-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride](/data/attachment/201705/28/36e5363f0c9f92378b75195743e2abb2.jpg) 9-ethyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11a-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride
9-ethyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11a-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride
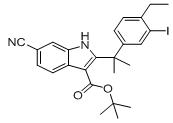 tert-butyl 6-cyano-2-(2-(4-ethyl-3-iodophenyl)propan-2-yl)-1H-indole-3-carboxylate
tert-butyl 6-cyano-2-(2-(4-ethyl-3-iodophenyl)propan-2-yl)-1H-indole-3-carboxylate
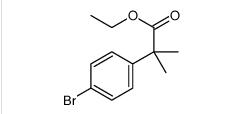 ethyl 2-(4-broMophenyl)-2-Methylpropanoate
ethyl 2-(4-broMophenyl)-2-Methylpropanoate
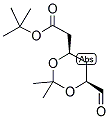 ert-Butyl (4R-cis)-6-formaldehydel-2,2-dimethyl-1,3-dioxane-4-acetate
ert-Butyl (4R-cis)-6-formaldehydel-2,2-dimethyl-1,3-dioxane-4-acetate
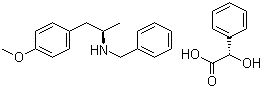 (2S)-Hydroxy(phenyl)acetic acid (2R)-N-benzyl-1-(4-methoxyphenyl)propan-2-amine
(2S)-Hydroxy(phenyl)acetic acid (2R)-N-benzyl-1-(4-methoxyphenyl)propan-2-amine
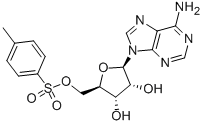 5-Tosyladenosine
5-Tosyladenosine
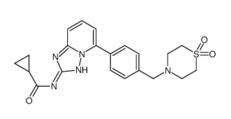 Filgotinib
Filgotinib
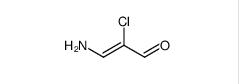 3-amino-2-chloroacrolein
3-amino-2-chloroacrolein
![2-Methylpyrazolo[1,5-a]pyrimidine-6-carboxamide 2-Methylpyrazolo[1,5-a]pyrimidine-6-carboxamide](/data/attachment/201706/03/2e19d959128718d26901f9909d7b9342.jpg) 2-Methylpyrazolo[1,5-a]pyrimidine-6-carboxamide
2-Methylpyrazolo[1,5-a]pyrimidine-6-carboxamide
![11-(1-Methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[d]iMidazo[1,2-a]azepine 11-(1-Methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[d]iMidazo[1,2-a]azepine](/data/attachment/201706/03/1549d9affee63ead337049001f25d9fa.jpg) 11-(1-Methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[d]iMidazo[1,2-a]azepine
11-(1-Methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[d]iMidazo[1,2-a]azepine
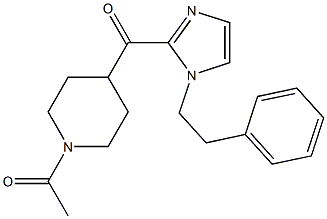 1-(4-(1-PHENETHYL-1H-IMIDAZOLE-2-CARBONYL)PIPERIDIN-1-YL)ETHANONE
1-(4-(1-PHENETHYL-1H-IMIDAZOLE-2-CARBONYL)PIPERIDIN-1-YL)ETHANONE
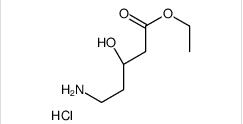 ethyl (3R)-5-amino-3-hydroxypentanoate,hydrochloride
ethyl (3R)-5-amino-3-hydroxypentanoate,hydrochloride
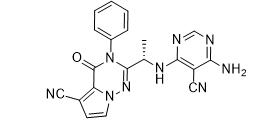 LAS191954 free base
LAS191954 free base
![tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate](/data/attachment/201706/03/8a3c0fcdeb9ed744fc854cf248d4d53e.jpg) tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate
tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate
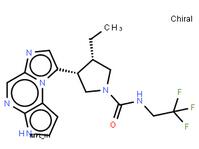 ABT-494 Intermeidate N-2
ABT-494 Intermeidate N-2
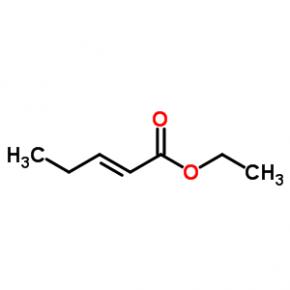 ethyl (2E)-pent-2-enoate
ethyl (2E)-pent-2-enoate
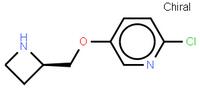 abt594 Intermediate
abt594 Intermediate
 LOXO101 Intermediate 2
LOXO101 Intermediate 2
 LOXO101 Intermediate 1
LOXO101 Intermediate 1
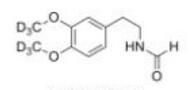 Deutetrabenazine intermediate N-2
Deutetrabenazine intermediate N-2
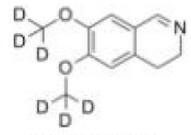 Deutetrabenazine intermediate N-1
Deutetrabenazine intermediate N-1
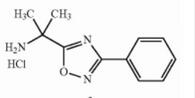 Naldemedine tosylate intermediate
Naldemedine tosylate intermediate
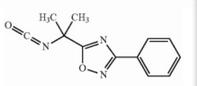 Naldemedine tosylate intermediate N-2
Naldemedine tosylate intermediate N-2
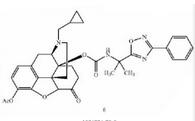 Naldemedine
Naldemedine
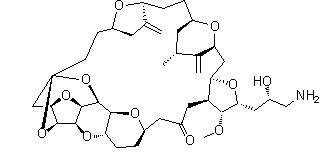 Eribulin
Eribulin
![2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]tetrahydro-4-methylene-, 2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]tetrahydro-4-methylene-,](/data/attachment/201706/03/3575f40dcc389832ca73cc99972a645b.gif.thumb.jpg) 2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]tetrahydro-4-methylene-,
2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]tetrahydro-4-methylene-,
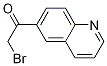 2-BroMo-1-quinolin-6-yl-ethanone
2-BroMo-1-quinolin-6-yl-ethanone
![6-[(6-Bromo-1H-[1,2,3]triazolo[4,5-b]pyrazin-1-yl)methyl]-7-fluoroquinoline 6-[(6-Bromo-1H-[1,2,3]triazolo[4,5-b]pyrazin-1-yl)methyl]-7-fluoroquinoline](/data/attachment/201706/07/27ae4307b53f4294590fb8f914894490.jpg) 6-[(6-Bromo-1H-[1,2,3]triazolo[4,5-b]pyrazin-1-yl)methyl]-7-fluoroquinoline
6-[(6-Bromo-1H-[1,2,3]triazolo[4,5-b]pyrazin-1-yl)methyl]-7-fluoroquinoline
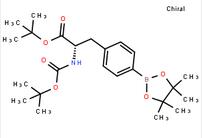 tert-butyl (S)-2-((tert-butoxycarbonyl)amino)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)propanoate
tert-butyl (S)-2-((tert-butoxycarbonyl)amino)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)propanoate
![7-Trifluoromethyl-imidazo[1,2-a]pyridine 7-Trifluoromethyl-imidazo[1,2-a]pyridine](/data/attachment/201706/07/24ba6100528abe0753ad9e82ef8dc810.gif) 7-Trifluoromethyl-imidazo[1,2-a]pyridine
7-Trifluoromethyl-imidazo[1,2-a]pyridine
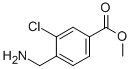 methyl 4-(aminomethyl)-3-chlorobenzoate
methyl 4-(aminomethyl)-3-chlorobenzoate
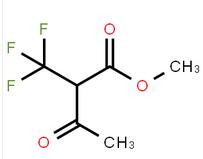 methyl 3-oxo-2-(trifluoromethyl)butanoate
methyl 3-oxo-2-(trifluoromethyl)butanoate
![2-AMino-4,5,6,7-tetrahydro-benzo[b]thiophene-3-carboxylic acid tert-butyl ester 2-AMino-4,5,6,7-tetrahydro-benzo[b]thiophene-3-carboxylic acid tert-butyl ester](/data/attachment/201706/07/22aadd4c55094254a681014935f56827.jpg) 2-AMino-4,5,6,7-tetrahydro-benzo[b]thiophene-3-carboxylic acid tert-butyl ester
2-AMino-4,5,6,7-tetrahydro-benzo[b]thiophene-3-carboxylic acid tert-butyl ester
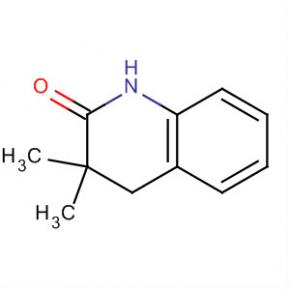 2(1H)-Quinolinone, 3,4-dihydro-3,3-dimethyl
2(1H)-Quinolinone, 3,4-dihydro-3,3-dimethyl
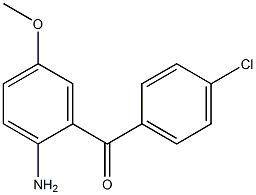 Methanone, (2-aMino-5-Methoxyphenyl)(4-chlorophenyl)
Methanone, (2-aMino-5-Methoxyphenyl)(4-chlorophenyl)
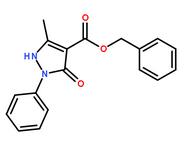 benzyl 5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxylate
benzyl 5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxylate
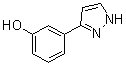 3-(1H-pyrazol-5-yl)phenol
3-(1H-pyrazol-5-yl)phenol
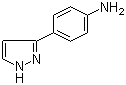 4-(1H-Pyrazol-3-yl)aniline
4-(1H-Pyrazol-3-yl)aniline
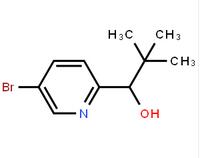 1-(5-bromo-pyridin-2-yl)-2,2-dimethyl-propan-1-ol
1-(5-bromo-pyridin-2-yl)-2,2-dimethyl-propan-1-ol
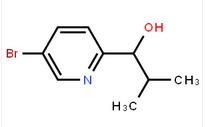 1-(5-bromo-pyridin-2-yl)-2-methyl-propan-1-ol
1-(5-bromo-pyridin-2-yl)-2-methyl-propan-1-ol
![Benzenemethanol, a-[(1S)-1-aminoethyl]-4-hydroxy-,(aR) Benzenemethanol, a-[(1S)-1-aminoethyl]-4-hydroxy-,(aR)](/data/attachment/201706/07/c4adcbada0ef372ae46cbaed643dd18e.jpg) Benzenemethanol, a-[(1S)-1-aminoethyl]-4-hydroxy-,(aR)
Benzenemethanol, a-[(1S)-1-aminoethyl]-4-hydroxy-,(aR)
![2(1H)-Quinolinone,5-[(1R)-2-amino-1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]ethyl]-8-hydroxy 2(1H)-Quinolinone,5-[(1R)-2-amino-1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]ethyl]-8-hydroxy](/data/attachment/201706/07/e0e9b5769a45af836d70be4140043125.gif.thumb.jpg) 2(1H)-Quinolinone,5-[(1R)-2-amino-1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]ethyl]-8-hydroxy
2(1H)-Quinolinone,5-[(1R)-2-amino-1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]ethyl]-8-hydroxy
![2-{[3-(4-Fluorophenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrim idin-2-yl]sulfanyl}-N-(6-methyl-1,3-benzothiazol-2-yl)acetamide 2-{[3-(4-Fluorophenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrim idin-2-yl]sulfanyl}-N-(6-methyl-1,3-benzothiazol-2-yl)acetamide](/data/attachment/201706/07/5754ee36bdfbf4148f45632422f563b9.jpg) 2-{[3-(4-Fluorophenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrim idin-2-yl]sulfanyl}-N-(6-methyl-1,3-benzothiazol-2-yl)acetamide
2-{[3-(4-Fluorophenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrim idin-2-yl]sulfanyl}-N-(6-methyl-1,3-benzothiazol-2-yl)acetamide
![2-((3-(2-methoxyphenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrimidin-2-yl)thio)-N-(6-methylbenzo[d]thiazol-2-yl)acetamide 2-((3-(2-methoxyphenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrimidin-2-yl)thio)-N-(6-methylbenzo[d]thiazol-2-yl)acetamide](/data/attachment/201706/08/47a8b3c98aef0b9ba378c4b7c6cef435.jpg) 2-((3-(2-methoxyphenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrimidin-2-yl)thio)-N-(6-methylbenzo[d]thiazol-2-yl)acetamide
2-((3-(2-methoxyphenyl)-4-oxo-3,4,6,7-tetrahydrothieno[3,2-d]pyrimidin-2-yl)thio)-N-(6-methylbenzo[d]thiazol-2-yl)acetamide
![N-(6-Methyl-2-benzothiazolyl)-2-[(3,4,6,7-tetrahydro-4-oxo-3-phenylthieno[3,2-d]pyrimidin-2-yl)thio]-acetamide N-(6-Methyl-2-benzothiazolyl)-2-[(3,4,6,7-tetrahydro-4-oxo-3-phenylthieno[3,2-d]pyrimidin-2-yl)thio]-acetamide](/data/attachment/201706/08/beda6f8f4655aa74d3646cfc7621fb20.jpg) N-(6-Methyl-2-benzothiazolyl)-2-[(3,4,6,7-tetrahydro-4-oxo-3-phenylthieno[3,2-d]pyrimidin-2-yl)thio]-acetamide
N-(6-Methyl-2-benzothiazolyl)-2-[(3,4,6,7-tetrahydro-4-oxo-3-phenylthieno[3,2-d]pyrimidin-2-yl)thio]-acetamide
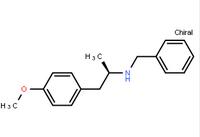 (R)-(-)-1-(4-methoxyphenyl)-2-benzylaminopropane
(R)-(-)-1-(4-methoxyphenyl)-2-benzylaminopropane
![4(3H)-Quinazolinone,7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl] 4(3H)-Quinazolinone,7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]](/data/attachment/201706/09/b600ffca12695094db2c5f6045cb6685.jpg) 4(3H)-Quinazolinone,7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]
4(3H)-Quinazolinone,7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]
![9-OXO-1,2,3,9-TETRAHYDRO-PYRROLO[2,1-B]QUINAZOLINE-6-CARBOXYLIC ACID 9-OXO-1,2,3,9-TETRAHYDRO-PYRROLO[2,1-B]QUINAZOLINE-6-CARBOXYLIC ACID](/data/attachment/201706/09/d6b395bbfb23e628be7d536d9cc2b512.gif) 9-OXO-1,2,3,9-TETRAHYDRO-PYRROLO[2,1-B]QUINAZOLINE-6-CARBOXYLIC ACID
9-OXO-1,2,3,9-TETRAHYDRO-PYRROLO[2,1-B]QUINAZOLINE-6-CARBOXYLIC ACID
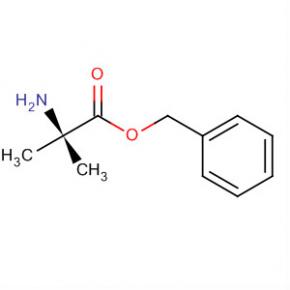 Alanine, 2-methyl-, phenylmethyl ester
Alanine, 2-methyl-, phenylmethyl ester
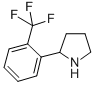 2-(2-TRIFLUOROMETHYL-PHENYL)-PYRROLIDINE
2-(2-TRIFLUOROMETHYL-PHENYL)-PYRROLIDINE
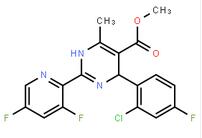 (-)-4(R)-(2-Chloro-4-fluorophenyl)-2-(3,5-difluoropyridin-2-yl)-6-methyl-1,4-dihydropyrimidine-5-carboxylic acid methyl ester
(-)-4(R)-(2-Chloro-4-fluorophenyl)-2-(3,5-difluoropyridin-2-yl)-6-methyl-1,4-dihydropyrimidine-5-carboxylic acid methyl ester
![2-Butenoic acid, 2-hydroxy-4-[5-(1-Methylethyl)-2,4-bis(phenylMethoxy)phenyl]-4-oxo-, ethyl ester 2-Butenoic acid, 2-hydroxy-4-[5-(1-Methylethyl)-2,4-bis(phenylMethoxy)phenyl]-4-oxo-, ethyl ester](/data/attachment/201706/09/11c6e17ba89840528c5461ae5350df33.gif) 2-Butenoic acid, 2-hydroxy-4-[5-(1-Methylethyl)-2,4-bis(phenylMethoxy)phenyl]-4-oxo-, ethyl ester
2-Butenoic acid, 2-hydroxy-4-[5-(1-Methylethyl)-2,4-bis(phenylMethoxy)phenyl]-4-oxo-, ethyl ester
methanone [4-amino-2-(ethylsulfanyl)pyrimidin-5-yl](2,3-difluoro-6-methoxyphenyl)methanone](/data/attachment/201706/09/ca947be16560699c92609cd96b352c02.png.thumb.jpg) [4-amino-2-(ethylsulfanyl)pyrimidin-5-yl](2,3-difluoro-6-methoxyphenyl)methanone
[4-amino-2-(ethylsulfanyl)pyrimidin-5-yl](2,3-difluoro-6-methoxyphenyl)methanone
![Benzenesulfonamide, 2-[(5-bromo-2-chloro-4-pyrimidinyl)amino]-N-methyl Benzenesulfonamide, 2-[(5-bromo-2-chloro-4-pyrimidinyl)amino]-N-methyl](/data/attachment/201706/10/ce0d621896c03bdb67e3b184103e84ff.png.thumb.jpg) Benzenesulfonamide, 2-[(5-bromo-2-chloro-4-pyrimidinyl)amino]-N-methyl
Benzenesulfonamide, 2-[(5-bromo-2-chloro-4-pyrimidinyl)amino]-N-methyl
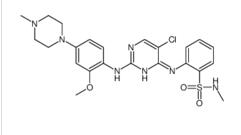 ALK inhibitor 2
ALK inhibitor 2
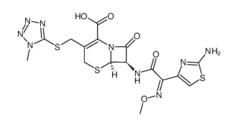 Cefmenoxime hydrochloride
Cefmenoxime hydrochloride
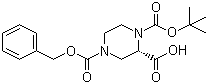 (S)-N-1-Boc-N-4-Cbz-2-piperazinecarboxylic acid
(S)-N-1-Boc-N-4-Cbz-2-piperazinecarboxylic acid
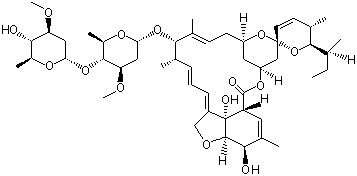 Avermectin
Avermectin
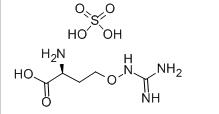 L-CANAVANINE SULFATE
L-CANAVANINE SULFATE
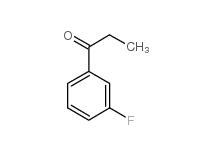 3-Fluoropropiophenone 455-67-4
3-Fluoropropiophenone 455-67-4
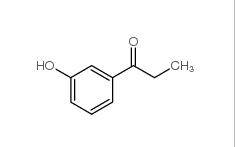 3-Hydroxypropiophenone 13103-80-5
3-Hydroxypropiophenone 13103-80-5
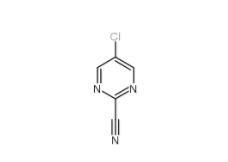 2-Cyano-5-chloropyrimidine 38275-56-8
2-Cyano-5-chloropyrimidine 38275-56-8
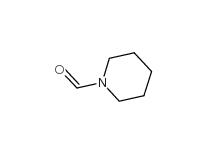 N-Formylpiperidine 2591-86-8
N-Formylpiperidine 2591-86-8
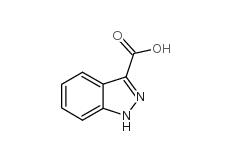 Indazole-3-carboxylic acid 4498-67-3
Indazole-3-carboxylic acid 4498-67-3
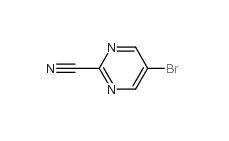 5-Bromo-2-cyanopyrimidine 38275-57-9
5-Bromo-2-cyanopyrimidine 38275-57-9
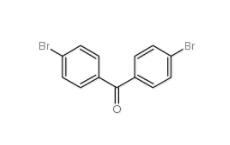 4,4-Dibromobenzophenone 3988-03-2
4,4-Dibromobenzophenone 3988-03-2
![1,2,3,4-Tetrahydro-benzo[b]azepin-5-one 1127-74-8 1,2,3,4-Tetrahydro-benzo[b]azepin-5-one 1127-74-8](/data/attachment/201901/28/b183df1e648eca4396ad0d319a1254bc.jpg) 1,2,3,4-Tetrahydro-benzo[b]azepin-5-one 1127-74-8
1,2,3,4-Tetrahydro-benzo[b]azepin-5-one 1127-74-8
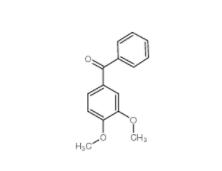 4038-14-6,(3,4-dimethoxyphenyl)-phenylmethanone 4038-14-6
4038-14-6,(3,4-dimethoxyphenyl)-phenylmethanone 4038-14-6
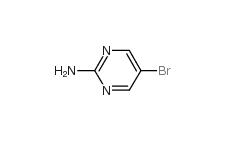 2-Amino-5-bromopyrimidine 7752-82-1
2-Amino-5-bromopyrimidine 7752-82-1
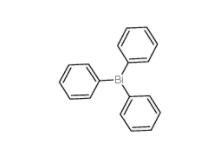 Triphenylbismuth 603-33-8
Triphenylbismuth 603-33-8
![3-Ethynylimidazo[1,2-a]pyridine 943320-53-4 3-Ethynylimidazo[1,2-a]pyridine 943320-53-4](/data/attachment/201901/28/d6294d1dabcee85ee04792b0c0e255c0.jpg) 3-Ethynylimidazo[1,2-a]pyridine 943320-53-4
3-Ethynylimidazo[1,2-a]pyridine 943320-53-4
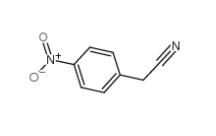 4-Nitrophenylacetonitrile 555-21-5
4-Nitrophenylacetonitrile 555-21-5
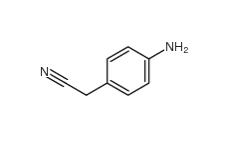 2-(4-aminophenyl)acetonitrile 3544-25-0
2-(4-aminophenyl)acetonitrile 3544-25-0
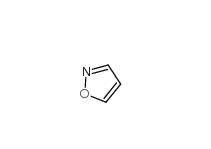 isoxazole 288-14-2
isoxazole 288-14-2
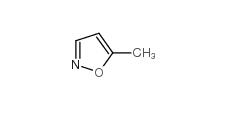 5-Methylisoxazole 5765-44-6
5-Methylisoxazole 5765-44-6
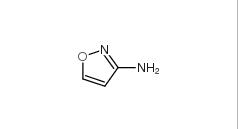 3-Aminoisoxazole 1750-42-1
3-Aminoisoxazole 1750-42-1
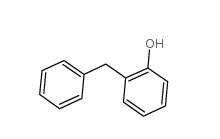 2-Hydroxydiphenylmethane 28994-41-4
2-Hydroxydiphenylmethane 28994-41-4
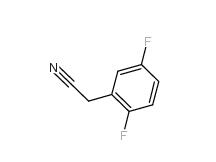 2,5-Difluorobenzyl Cyanide 69584-87-8
2,5-Difluorobenzyl Cyanide 69584-87-8
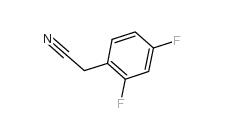 2,4-Difluorophenylacetonitrile 656-35-9
2,4-Difluorophenylacetonitrile 656-35-9
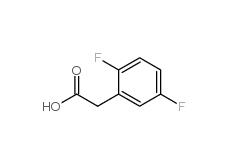 2,5-Difluorophenylacetic acid 85068-27-5
2,5-Difluorophenylacetic acid 85068-27-5
 2,4-Difluorophenylacetic acid 81228-09-3
2,4-Difluorophenylacetic acid 81228-09-3
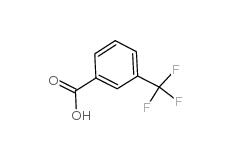 3-trifluoromethylbenzoic acid 454-92-2
3-trifluoromethylbenzoic acid 454-92-2
![2-[3-(trifluoromethyl)phenyl]acetonitrile 2338-76-3 2-[3-(trifluoromethyl)phenyl]acetonitrile 2338-76-3](/data/attachment/201901/29/22b99245cb0bbcd2d86f238725d9fb9d.jpg) 2-[3-(trifluoromethyl)phenyl]acetonitrile 2338-76-3
2-[3-(trifluoromethyl)phenyl]acetonitrile 2338-76-3
![2-[3-(trifluoromethyl)phenyl]acetic acid 351-35-9 2-[3-(trifluoromethyl)phenyl]acetic acid 351-35-9](/data/attachment/201901/29/7dbc74a276a4c124b9460222442fd80f.jpg) 2-[3-(trifluoromethyl)phenyl]acetic acid 351-35-9
2-[3-(trifluoromethyl)phenyl]acetic acid 351-35-9
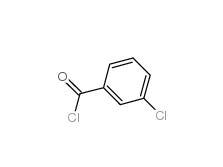 3-Chlorobenzoyl chloride 618-46-2
3-Chlorobenzoyl chloride 618-46-2
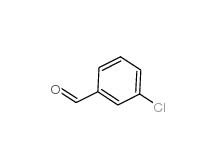 3-Chlorobenzaldehyde 587-04-2
3-Chlorobenzaldehyde 587-04-2
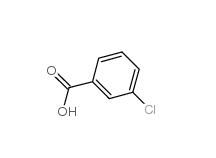 3-chlorobenzoic acid 535-80-8
3-chlorobenzoic acid 535-80-8
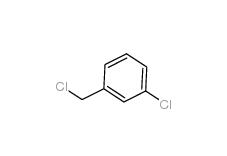 3-Chlorobenzyl chloride 620-20-2
3-Chlorobenzyl chloride 620-20-2
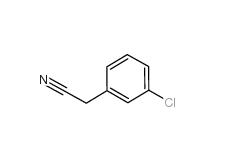 3-Chlorobenzyl cyanide 1529-41-5
3-Chlorobenzyl cyanide 1529-41-5
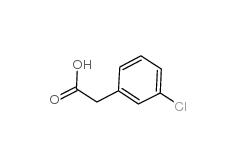 2-(3-chlorophenyl)acetic acid 1878-65-5
2-(3-chlorophenyl)acetic acid 1878-65-5
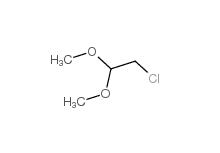 Dimethylchloroacetal 97-97-2
Dimethylchloroacetal 97-97-2
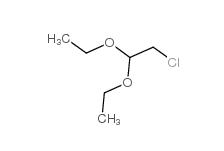 Chloroacetaldehyde diethyl acetal 621-62-5
Chloroacetaldehyde diethyl acetal 621-62-5
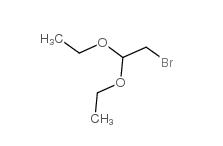 2-bromo-1,1-diethoxyethane 2032-35-1
2-bromo-1,1-diethoxyethane 2032-35-1
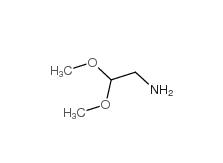 2,2-dimethoxyethanamine 22483-09-6
2,2-dimethoxyethanamine 22483-09-6
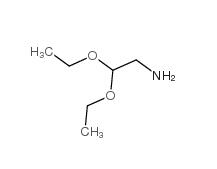 2,2-Diethoxyethylamine 645-36-3
2,2-Diethoxyethylamine 645-36-3
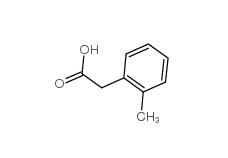 2-Methylphenylacetic acid 644-36-0
2-Methylphenylacetic acid 644-36-0
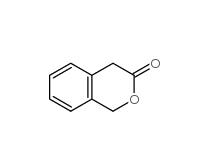 3-Isochromanone 4385-35-7
3-Isochromanone 4385-35-7
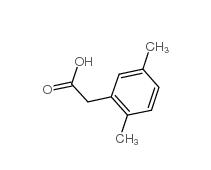 2,5-Dimethylphenylacetic acid 13612-34-5
2,5-Dimethylphenylacetic acid 13612-34-5
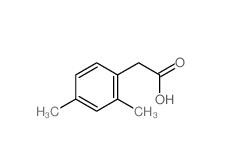 2,4-Dimethylphenylacetic Acid 6331-04-0
2,4-Dimethylphenylacetic Acid 6331-04-0
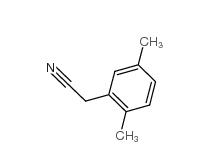 2,5-Dimethylphenylacetonitrile 16213-85-7
2,5-Dimethylphenylacetonitrile 16213-85-7
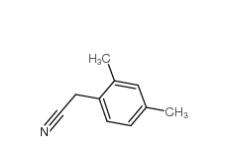 2,4-Dimethylphenylacetonitrile 68429-53-8
2,4-Dimethylphenylacetonitrile 68429-53-8
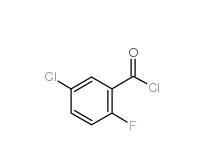 5-CHLORO-2-FLUOROBENZOYL CHLORIDE 394-29-6
5-CHLORO-2-FLUOROBENZOYL CHLORIDE 394-29-6
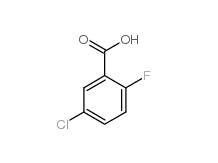 5-Chloro-2-fluorobenzoic acid 394-30-9
5-Chloro-2-fluorobenzoic acid 394-30-9
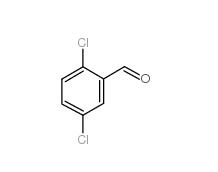 2,5-Dichlorobenzaldehyde 6361-23-5
2,5-Dichlorobenzaldehyde 6361-23-5
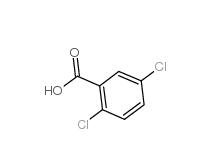 2,5-Dichlorobenzoic acid 50-79-3
2,5-Dichlorobenzoic acid 50-79-3
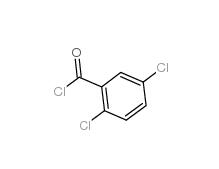 2,5-DICHLOROBENZOYL CHLORIDE 2905-61-5
2,5-DICHLOROBENZOYL CHLORIDE 2905-61-5
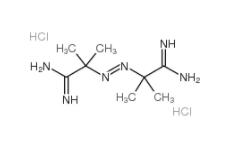 2,2-Azobis(2-methylpropionamidine) dihydrochloride 2997-92-4
2,2-Azobis(2-methylpropionamidine) dihydrochloride 2997-92-4
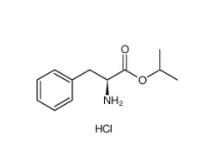 L-Phenylalanine, 1-methylethyl ester, hydrochloride 95585-78-7
L-Phenylalanine, 1-methylethyl ester, hydrochloride 95585-78-7
 Diphenylphosphoryl azide 26386-88-9
Diphenylphosphoryl azide 26386-88-9
 Methyl 4-(bromomethyl)benzoate 2417-72-3
Methyl 4-(bromomethyl)benzoate 2417-72-3
 Tideglusib 865854-05-3
Tideglusib 865854-05-3
 Disodium 7,7-(carbonyldiimino)bis(4-hydroxynaphthalene-2-sulphonate) 20324-87-2
Disodium 7,7-(carbonyldiimino)bis(4-hydroxynaphthalene-2-sulphonate) 20324-87-2
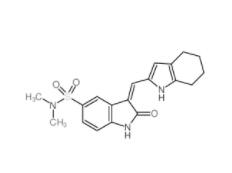 SU 6656 330161-87-0
SU 6656 330161-87-0
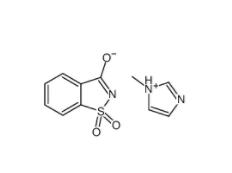 Saccharin 1-methylimidazole 482333-74-4
Saccharin 1-methylimidazole 482333-74-4
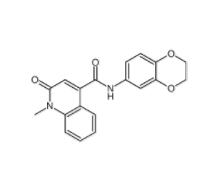 CeMMEC13 1790895-25-8
CeMMEC13 1790895-25-8
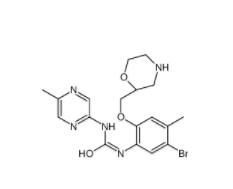 Rabusertib 911222-45-2
Rabusertib 911222-45-2
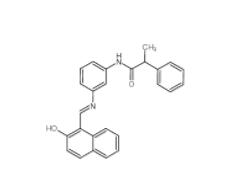 Salermide 1105698-15-4
Salermide 1105698-15-4
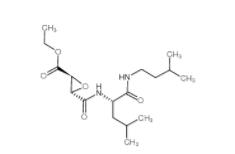 EST 88321-09-9
EST 88321-09-9
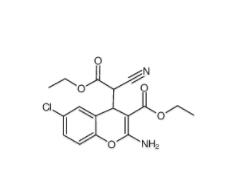 SC79 305834-79-1
SC79 305834-79-1
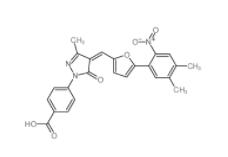 C646 328968-36-1
C646 328968-36-1
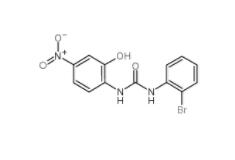 1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea 182498-32-4
1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea 182498-32-4
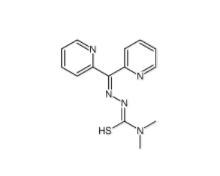 Dp44mT 152095-12-0
Dp44mT 152095-12-0
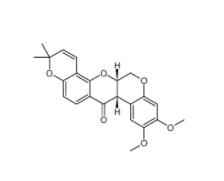 Deguelin 522-17-8
Deguelin 522-17-8
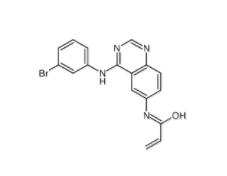 PD168393 194423-15-9
PD168393 194423-15-9
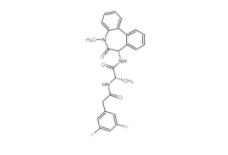 YO01027 209984-56-5
YO01027 209984-56-5
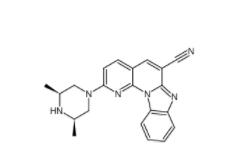 DC10539 1822358-25-7
DC10539 1822358-25-7
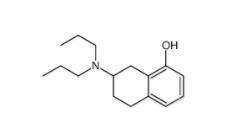 8-OH-DPAT 78950-78-4
8-OH-DPAT 78950-78-4
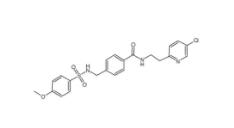 YU238259 1943733-16-1
YU238259 1943733-16-1
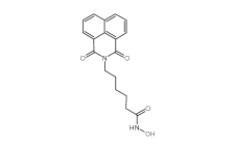 Scriptaid 287383-59-9
Scriptaid 287383-59-9
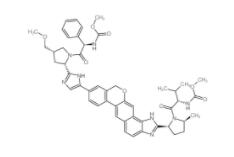 Velpatasvir 1377049-84-7
Velpatasvir 1377049-84-7
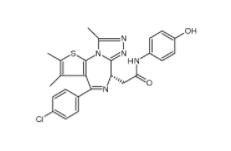 OTX015 202590-98-5
OTX015 202590-98-5
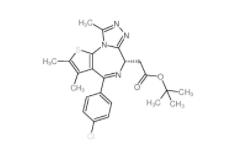 (+)-JQ-1 1268524-70-4
(+)-JQ-1 1268524-70-4
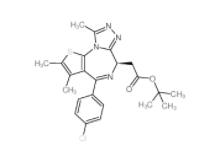 (-)-JQ-1 1268524-71-5
(-)-JQ-1 1268524-71-5
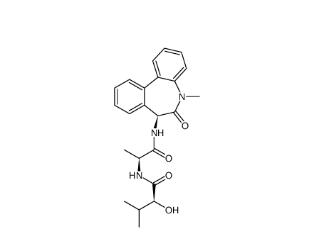 LY 900009 209984-68-9
LY 900009 209984-68-9
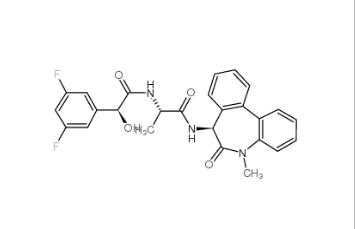 LY-411575 209984-57-6
LY-411575 209984-57-6
![(4-nitrophenyl) 4-[bis(1,3-benzodioxol-5-yl)-hydroxymethyl]piperidine-1-carboxylate 1101854-58-3 (4-nitrophenyl) 4-[bis(1,3-benzodioxol-5-yl)-hydroxymethyl]piperidine-1-carboxylate 1101854-58-3](/data/attachment/201903/22/9e81dae7e0bdec56ac6052b1872d9626.jpg) (4-nitrophenyl) 4-[bis(1,3-benzodioxol-5-yl)-hydroxymethyl]piperidine-1-carboxylate 1101854-58-3
(4-nitrophenyl) 4-[bis(1,3-benzodioxol-5-yl)-hydroxymethyl]piperidine-1-carboxylate 1101854-58-3
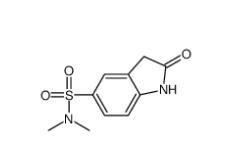 N,N-dimethyl-2-oxo-1,3-dihydroindole-5-sulfonamide 170565-89-6
N,N-dimethyl-2-oxo-1,3-dihydroindole-5-sulfonamide 170565-89-6
![5,6,11,12-tetrahydrodibenzo[1,2-b:1,2-g][8]annulene 1460-59-9 5,6,11,12-tetrahydrodibenzo[1,2-b:1,2-g][8]annulene 1460-59-9](/data/attachment/201903/23/50930df6d55412ac8f4da0724b497aaf.jpg) 5,6,11,12-tetrahydrodibenzo[1,2-b:1,2-g][8]annulene 1460-59-9
5,6,11,12-tetrahydrodibenzo[1,2-b:1,2-g][8]annulene 1460-59-9
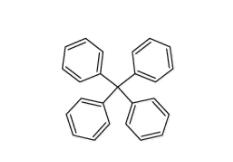 Tetraphenylmethane 630-76-2
Tetraphenylmethane 630-76-2
![2-chloro-6-[(2R)-2-hydroxy-3-[(2-methyl-1-naphthalen-2-ylpropan-2-yl)amino]propoxy]benzonitrile 284035-33-2 2-chloro-6-[(2R)-2-hydroxy-3-[(2-methyl-1-naphthalen-2-ylpropan-2-yl)amino]propoxy]benzonitrile 284035-33-2](/data/attachment/201903/23/7e63bafe6c4b7e146e00c57dfca99672.jpg) 2-chloro-6-[(2R)-2-hydroxy-3-[(2-methyl-1-naphthalen-2-ylpropan-2-yl)amino]propoxy]benzonitrile 284035-33-2
2-chloro-6-[(2R)-2-hydroxy-3-[(2-methyl-1-naphthalen-2-ylpropan-2-yl)amino]propoxy]benzonitrile 284035-33-2
 1616380-54-1
1616380-54-1
![N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-methyl-1-oxo-1,2-dihydroisoquinoline-4-carboxamide 440662-09-9 N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-methyl-1-oxo-1,2-dihydroisoquinoline-4-carboxamide 440662-09-9](/data/attachment/201903/23/e26baac537719657acd9f1f55568401d.jpg) N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-methyl-1-oxo-1,2-dihydroisoquinoline-4-carboxamide 440662-09-9
N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-methyl-1-oxo-1,2-dihydroisoquinoline-4-carboxamide 440662-09-9
![N-[2-[2-(4-chlorophenyl)-1,3-thiazol-4-yl]ethyl]butanamide 932986-18-0 N-[2-[2-(4-chlorophenyl)-1,3-thiazol-4-yl]ethyl]butanamide 932986-18-0](/data/attachment/201903/23/7d2bbd100c8322ae16168937617e1bb2.jpg) N-[2-[2-(4-chlorophenyl)-1,3-thiazol-4-yl]ethyl]butanamide 932986-18-0
N-[2-[2-(4-chlorophenyl)-1,3-thiazol-4-yl]ethyl]butanamide 932986-18-0
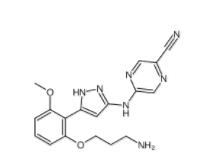 5-(5-(2-(3-aminopropoxy)-6-methoxyphenyl)-1H-pyrazol-3-ylamino)pyrazine-2-carbonitrile 1234015-52-1
5-(5-(2-(3-aminopropoxy)-6-methoxyphenyl)-1H-pyrazol-3-ylamino)pyrazine-2-carbonitrile 1234015-52-1
![3-Methyl-3-[(1E)-2-phenylethenyl]-3,2:5,2:5,3-quaterpyridine 1651890-44-6 3-Methyl-3-[(1E)-2-phenylethenyl]-3,2:5,2:5,3-quaterpyridine 1651890-44-6](/data/attachment/201903/23/07bf6fd99e81033df0c83039ccdde036.jpg) 3-Methyl-3-[(1E)-2-phenylethenyl]-3,2:5,2:5,3-quaterpyridine 1651890-44-6
3-Methyl-3-[(1E)-2-phenylethenyl]-3,2:5,2:5,3-quaterpyridine 1651890-44-6
![3-[4-(Dimethylamino)-3-biphenylyl]-1,1-dimethylure 1469924-27-3 3-[4-(Dimethylamino)-3-biphenylyl]-1,1-dimethylure 1469924-27-3](/data/attachment/201903/23/f69ad7342d131146640e0c88f73e9a25.jpg) 3-[4-(Dimethylamino)-3-biphenylyl]-1,1-dimethylure 1469924-27-3
3-[4-(Dimethylamino)-3-biphenylyl]-1,1-dimethylure 1469924-27-3
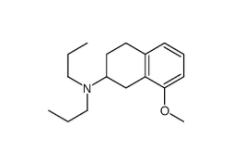 8-Methoxy-N,N-dipropyl-1,2,3,4-tetrahydro-2-naphthalenamine 3897-94-7
8-Methoxy-N,N-dipropyl-1,2,3,4-tetrahydro-2-naphthalenamine 3897-94-7
![4-{4-[(4-{[3-(Acryloylamino)phenyl]amino}-5-fluoro-2-pyrimidinyl) amino]phenoxy}-N-methyl-2-pyridinecarboxamide 1202759-32-7 4-{4-[(4-{[3-(Acryloylamino)phenyl]amino}-5-fluoro-2-pyrimidinyl) amino]phenoxy}-N-methyl-2-pyridinecarboxamide 1202759-32-7](/data/attachment/201903/23/bb4110673d0676f81860d708092eb660.jpg) 4-{4-[(4-{[3-(Acryloylamino)phenyl]amino}-5-fluoro-2-pyrimidinyl) amino]phenoxy}-N-methyl-2-pyridinecarboxamide 1202759-32-7
4-{4-[(4-{[3-(Acryloylamino)phenyl]amino}-5-fluoro-2-pyrimidinyl) amino]phenoxy}-N-methyl-2-pyridinecarboxamide 1202759-32-7
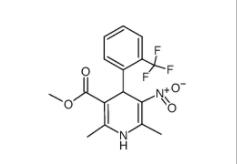 1,4-Dihydro-2,6-dimethyl-3-nitro-4-(2-trifluoromethylphenyl)-pyridine-5-carboxylic acid methyl ester 71145-03-4
1,4-Dihydro-2,6-dimethyl-3-nitro-4-(2-trifluoromethylphenyl)-pyridine-5-carboxylic acid methyl ester 71145-03-4
![2-[[3-[[2-(dimethylamino)phenyl]methyl]-2-pyridin-4-yl-1,3-diazinan-1-yl]methyl]-N,N-dimethylaniline 500579-04-4 2-[[3-[[2-(dimethylamino)phenyl]methyl]-2-pyridin-4-yl-1,3-diazinan-1-yl]methyl]-N,N-dimethylaniline 500579-04-4](/data/attachment/201903/23/b396a2326dddb511aae497b01fbd4c77.jpg) 2-[[3-[[2-(dimethylamino)phenyl]methyl]-2-pyridin-4-yl-1,3-diazinan-1-yl]methyl]-N,N-dimethylaniline 500579-04-4
2-[[3-[[2-(dimethylamino)phenyl]methyl]-2-pyridin-4-yl-1,3-diazinan-1-yl]methyl]-N,N-dimethylaniline 500579-04-4
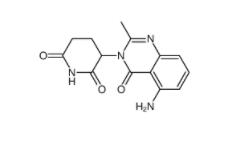 CC-122 1015474-32-4
CC-122 1015474-32-4
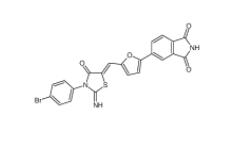 Bioymifi 1420071-30-2
Bioymifi 1420071-30-2
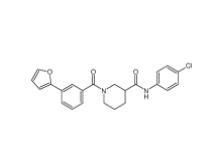 N-(4-chlorophenyl)-1-(3-(furan-2-yl)benzoyl)piperidine-3-carboxamide 1443437-74-8
N-(4-chlorophenyl)-1-(3-(furan-2-yl)benzoyl)piperidine-3-carboxamide 1443437-74-8
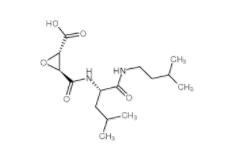 E-64C 76684-89-4
E-64C 76684-89-4
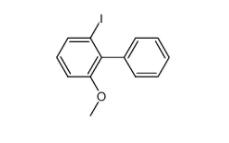 2-iodo-6-methoxybiphenyl 84253-78-1
2-iodo-6-methoxybiphenyl 84253-78-1
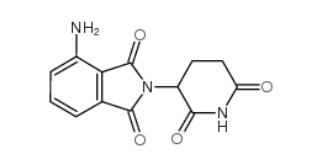 pomalidomide 19171-19-8
pomalidomide 19171-19-8
 4EP-Directory listing
4EP-Directory listing
 Stearoylbenzoylmethane 58446-52-9
Stearoylbenzoylmethane 58446-52-9
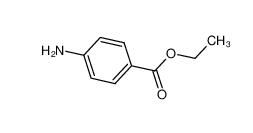 benzocaine 94-09-7
benzocaine 94-09-7
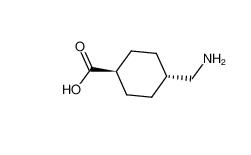 tranexamic acid 1197-18-8
tranexamic acid 1197-18-8
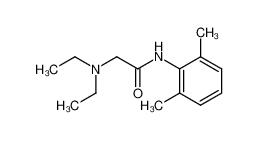 lidocaine 137-58-6
lidocaine 137-58-6
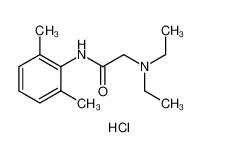 lidocaine hydrochloride 73-78-9
lidocaine hydrochloride 73-78-9
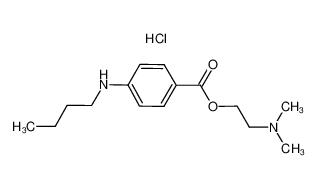 Tetracaine hydrochloride 136-47-0
Tetracaine hydrochloride 136-47-0
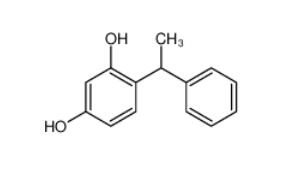 4-(1-phenylethyl)benzene-1,3-diol 85-27-8
4-(1-phenylethyl)benzene-1,3-diol 85-27-8
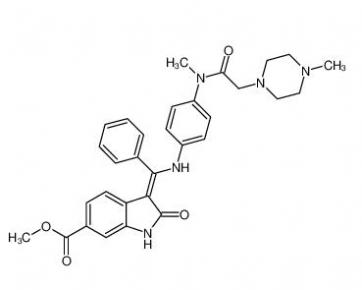 Nintedanib 656247-17-5
Nintedanib 656247-17-5
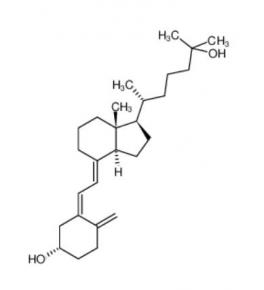 calcidiol 19356-17-3
calcidiol 19356-17-3
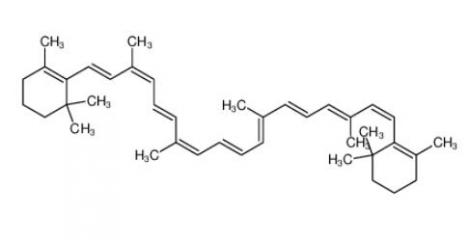 β-carotene 7235-40-7
β-carotene 7235-40-7
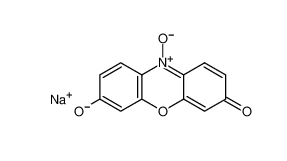 Resazurin sodium salt 62758-13-8
Resazurin sodium salt 62758-13-8
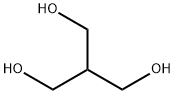 4704-94-3 2-(HYDROXYMETHYL)-1,3-PROPANEDIOL
4704-94-3 2-(HYDROXYMETHYL)-1,3-PROPANEDIOL
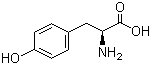 L-Tyrosine 60-18-4
L-Tyrosine 60-18-4
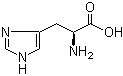 L-Histidine 71-00-1
L-Histidine 71-00-1
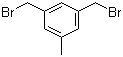 3,5-Bis(bromomethyl)toluene 19294-04-3
3,5-Bis(bromomethyl)toluene 19294-04-3
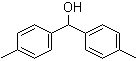 Bis(4-methylphenyl)methanol 885-77-8
Bis(4-methylphenyl)methanol 885-77-8
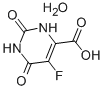 5-Fluoroorotic Acid Hydrate 207291-81-4
5-Fluoroorotic Acid Hydrate 207291-81-4
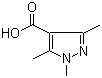 1,3,5-Trimethyl-1H-pyrazole-4-carboxylic acid 1125-29-7
1,3,5-Trimethyl-1H-pyrazole-4-carboxylic acid 1125-29-7
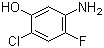 5-Amino-2-chloro-4-fluorophenol 84478-72-8
5-Amino-2-chloro-4-fluorophenol 84478-72-8
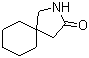 Gabapentin-lactam 64744-50-9
Gabapentin-lactam 64744-50-9
 1EP-Directory listing
1EP-Directory listing
![[2-(aminocarbonyl)phenyl]acetic acid 23362-56-3 [2-(aminocarbonyl)phenyl]acetic acid 23362-56-3](/data/attachment/202211/10/9756043560e11c17cf958f3ed54d541a.png.thumb.jpg) [2-(aminocarbonyl)phenyl]acetic acid 23362-56-3
[2-(aminocarbonyl)phenyl]acetic acid 23362-56-3
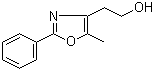 2-(5-Methyl-2-phenyl-1,3-oxazol-4-yl)ethan-1-ol 103788-65-4
2-(5-Methyl-2-phenyl-1,3-oxazol-4-yl)ethan-1-ol 103788-65-4
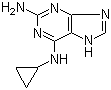 2-Amino-6-cyclopropylamino-9H-purine 120503-69-7
2-Amino-6-cyclopropylamino-9H-purine 120503-69-7
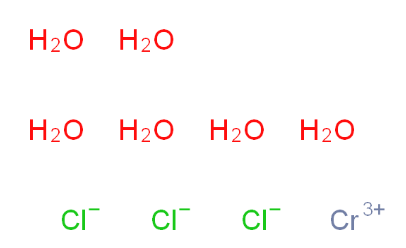 Chromic chloride hexahydrate 10060-12-5
Chromic chloride hexahydrate 10060-12-5
 2EP-Directory listing 2
2EP-Directory listing 2
 3EP-Directory listing 3
3EP-Directory listing 3